Abstract
Pelgipeptins are cyclic lipopeptides composed of nine amino acids and a short fatty acid chain. In the present study, we report a novel pelgipeptin peptide that was isolated from Paenibacillus elgii BC34-6 and named pelgipeptin E (PGP-E). The molecular mass of PGP-E was 1072 Da as determined by liquid chromatography-mass spectrometry and the amino acid sequence was elucidated by tandem mass spectrometry. The complete molecular structure of PGP-E was characterized using 2D NMR spectroscopy. PGP-E consisted of a cyclic peptide backbone of Dab1-Val2-Dab3-Phe4-Leu5-Dab6-Val7-Leu8-Ser9 and a lipid chain (-CH2CH2CH3). PGP-E had broad antimicrobial activity against gram-negative and -positive bacteria, including methicillin-resistant Staphylococcus aureus strains. Furthermore, the mode of action of PGP-E was investigated using calcein dye leakage and membrane depolarization assays, which suggest that PGP-E acts via a membrane-active mechanism. The hemolytic activity of PGP-E was significantly lower than that of melittin, a well-known membrane-active peptide derived from bee venom. These results suggest that PGP-E is a potential candidate in the development of new peptide antibiotics.
Similar content being viewed by others
Introduction
Bacterial pathogens are the leading cause of hospital-acquired infections and are often resistant to most typically used antibiotics due to their acquisition of resistance genes and ability to form biofilms [1, 2]. Although limited therapeutic options are presently available, new and innovative antibiotics, preferably with novel modes of action and/or using novel types of drugs, are needed to provide effective treatments. Among natural antibiotics, cyclic lipopeptides (CLPs) are a promising new class of antibacterial agents, especially against multidrug-resistant pathogens [3,4,5]. CLP antibiotics, such as polymyxin and daptomycin, are generally composed of a hydrophobic fatty acid tail linked to a peptide that is cyclized by a lactone or peptide bond between two amino acids or an amino acid and a fatty acid moiety. The structural diversity of CLPs arises from differences in the length and composition of the fatty acid chains and the amino acid sequences [6,7,8]. Most natural CLPs are secondary metabolites of soil-borne bacteria in the Streptomyces, Bacillus, and Paenibacillus genera [9, 10].
The endospore-forming bacteria Paenibacillus are a genus of facultative anaerobes that were originally included within the genus Bacillus, and reclassified as a separate genus in 1993 after extensive comparative analysis of 16S ribosomal RNA (rRNA) sequences [11]. Bacteria belonging to this genus have been detected in a variety of environments, including soil, water, rhizosphere, and clinical samples [12,13,14,15]. For instance, Paenibacillus elgii B69 was first isolated from mountain soil, from the Tianmu Mountain National Reserve in Hangzhou in China. It possesses potent antimicrobial activity against a wide range of pathogens [16]. To date, pelgipeptin (PGP)-A, -B, -C, and -D (Fig. 1) have been isolated from P. elgii B69, which have molecular masses of 1072.6, 1100.7, 1086.6, and 1086.6Da, respectively [16, 17]. PGPs are composed of a cyclic peptide backbone with nine amino acids and a short lipid chain. These molecules display broad-spectrum antimicrobial activity against pathogenic bacteria, including methicillin-resistant Staphylococcus aureus (MRSA) and fungi, including Fusarium oxysporum and Colletotrichum lini [16, 17].
In the present study, we isolated a new antimicrobial compound from a Paenibacillus strain found in red clay soil, which was identified as P. elgii BC34-6 and produced PGP peptides. Using antimicrobial assays and structural analysis, we identified a novel PGP peptide from the culture media of this strain and named it PGP-E. PGP-E was found to have antimicrobial activity against various bacteria, including MRSA, with maximum inhibitory concentrations (MICs) of 2–8 µg ml−1. We further investigated the mode of action of PGP-E using fluorescence experiments and hemolysis assays. The newly isolated PGP-E had high antimicrobial activity, but low hemolytic activity, suggesting that PGP-E is a potential antibiotic and that the BC34-6 strain may be utilized for PGP peptide production.
Materials and methods
Bacterial strains
The BC34-6 strain was isolated from red clay soil samples collected from Jeonnam Province in South Korea. Soil samples (1 g) were suspended in 9 ml sterilized distilled water. The soil suspensions were serially diluted 10-fold and spread onto tryptic soy agar plates, which were incubated at 37°C for 24 h. The bacteria were maintained in tryptic soy broth (17 g pancreatic digest of casein, 3 g papaic digest of soybean, 2.5 g dextrose, 5 g sodium chloride, and 2.5 g dipotassium phosphate per 1 liter) and stored at −70°C as 20% glycerol stocks. Growth inhibition of Escherichia coli by bacterial isolates was tested on tryptic soy agar plates. The BC34-6 strain exhibited the strongest antimicrobial activity, and was selected for further study.
Identification of BC34-6 strain
The BC34-6 strain was identified using the molecular phylogenetic method of comparing 16S rRNA gene sequences. BC34-6 was incubated in tryptic soy media at 37°C for 20 h. The cells were collected and lysed in 100 µl lysis buffer (0.4 M NaCl, 0.2 M Tris-HCl, 0.01 M EDTA, 1% SDS, pH 7.6). Tris-EDTA buffer (pH 8.0, 200 µl) and phenol/chloroform were added to the tubes containing the samples, which were then mixed gently. The mixtures were centrifuged at 17,226× g for 5 min. Next, 5 µl of RNase A (Promega, USA) was added to the supernatant, and the samples were incubated at 37°C for 1 h. Chloroform was added to the DNA layer, which was then centrifuged at 17,226× g for 5 min. The resulting supernatants were washed twice with 70% ethanol and dried using a SpeedVac (Hanil, South Korea). The extracted genomic DNA samples were stored at −20 ℃ until further use. Primers 27F (5′GTTTGATCCTGGCTCAG-3′) and 1489R (5′-TACCTTGTTACGACTTCA-3′) were used for 16S rRNA analysis. PCR mixtures contained 2 U Taq polymerase, 50 µl 1.5 mM MgCl2 (Promega, USA), 10 mM dNTPs (Promega, USA), 50 µl of each primer (10 mM), and 100 ng of DNA template. The PCR cycles consisted of an initial denaturation step of 95°C for 5 min, 30 cycles of 95°C for 1 min, 53°C for 40 or 60S, and 72°C for 1 min, and then a final elongation step of 72°C for 10 min.
Culturing of BC34-6 strain
The BC34-6 strain was incubated in tryptic soy broth at 30°C with shaking at 150 rpm for 48 h, and used to inoculate M9 media (6 g sodium hydrogen phosphate, 3 g potassium dihydrogen phosphate, 1 g ammonium chloride, 0.5 g sodium chloride, 1% sucrose, and 0.5% yeast extract per 1 liter), which was then incubated at 30°C with shaking at 150 rpm for 5 d.
Purification of PGP-E
The overall procedure for PGP-E purification is summarized in Fig. 2. The culture broth was centrifuged at 4600× g for 30 min. The supernatant was loaded onto a column of Diaion HP-20 (IONTEC, South Korea). The column was washed with distilled water and the samples were eluted using a stepwise ethanol gradient (20, 40, 60, 80, and then 100% v/v) and lyophilized. The resulting crude samples were dissolved in 100% methanol and further purified by preparative reversed-phase high-performance liquid chromatography (RP-HPLC) using a Shim-packed C18 column (20 × 250 mm, Shimadzu, Japan). The mobile phases were distilled water and acetonitrile (ACN) containing 0.05% trifluoroacetic acid (TFA). A binary gradient of 30–46% over 25 min was used for RP-HPLC purification with a flow rate of 10 ml min−1. Elution was monitored by measuring the absorbance at 230 nm.
Liquid chromatography tandem mass spectrometry analysis
The lactone ring of the purified PGP-E (1 mg) was completely hydrolyzed with 1 M KOH (100 µl) at 20°C for 3 min. The reaction mixture was diluted with 2 ml of 1% TFA in water to stop the reaction. The resulting hydrolysate was applied to a Sep-pak C18 cartridge (Waters, USA) for buffer exchange. After loading the sample, the cartridge was washed with 5 ml distilled water. Linear PGP-E was eluted with 95% ACN containing 0.05% TFA. The eluent was concentrated and dissolved in 50% ACN. The amino acid sequence of PGP-E was determined using electrospray ionization tandem mass spectrometry (ESI-MS/MS). MS/MS was analyzed using an API2000 (AB SCIEX, USA) in positive ion mode.
NMR analysis
NMR samples were prepared by dissolving the lyophilized PGP-E peptide (6.7 mM) in DMSO-d6. All NMR spectra were recorded at 25°C using a Bruker Avance 600 MHz NMR spectrometer (Bruker Biospin, Germany). Chemical shifts are shown with respect to the DMSO-d6 residual signals at 2.6 and 43.0 ppm for 1H and 13C spectra, respectively. The 1H and 13C NMR assignments were supported by total correlation spectroscopy (TOCSY), double-quantum-filtered (DQF)-COSY, NOESY, heteronuclear single quantum correlation (HSQC), and heteronuclear multiple-bond correlation (HMBC) experiments.
Determination of MICs
Bacteria, including E. coli (KCTC 1682), Staphylococcus typhimurium (KCTC 1926), Pseudomonas aeruginosa (KCTC 1637), Bacillus subtilis (KCTC 3068), Staphylococcus epidermidis (KCTC 1917), and S. aureus (KCTC 1621) were procured from the Korean Collection for Type Cultures (KCTC) at the Korea Research Institute of Bioscience and Biotechnology (Deajeon, South Korea). MRSA strains (CCARM 3089, CCARM 3090, and CCARM 3095) were obtained from the Culture Collection of Antibiotic-Resistant Microbes (CCARM) at the Seoul Women’s University (Seoul, South Korea). All bacteria were routinely grown at 37°C in Luria-Bertani broth. MICs were measured in 96-well microplates using the micro-broth dilution method. Bacteria were grown in Luria-Bertani broth at 37°C for 18 h and then diluted with 1% peptone to a final concentration of 2 × 106 colony-forming units per ml. Subsequently, 100 µl of a bacterial suspension and 100 µl of peptide solution were mixed together in a 96-well plate. The peptide solution was prepared by performing two-fold dilutions in 1% peptone, while the PGP-E solutions were prepared to final peptide concentrations of 0.06–128 µg ml−1. The bacteria and peptide solution mixtures were incubated at 37°C for 18 h. The peptide MIC was defined as the lowest concentration of peptide that inhibited bacterial growth.
Hemolytic activity
Peptide hemolytic activity was measured using human red blood cells (HRBCs). Fresh cells were washed three times with phosphate buffered saline (PBS) by centrifuging for 10 min at 1000× g and resuspended in PBS. Different concentrations (0.01–200 µM) of peptides were added to 4% HRBCs in PBS, which were then incubated at 37°C for 1 h. After the mixtures were centrifuged at 1000× g for 5 min, the aliquots of the supernatant were transferred to 96-well plates. Hemolytic activity was measured based on absorbance at 405 nm with a microplate reader (EL800, Bio-Tek Instruments, VT, USA). PBS and Triton X-100 were used as negative and positive controls, respectively. The percent hemolysis was calculated using the following equation: Hemolysis (%) = (Abs in peptide − Abs in PBS) / (Abs in Triton X-100 − Abs in PBS)] × 100, where Abs represents absorbance.
Calcein-release experiments
Liposomes were prepared by vortexing the required amount of EYPE/EYPG (7:3 w/w). The lipids were dissolved in 100% methanol and dried and the solvents were removed to generate a lipid film. Any remaining trace amounts of organic solvent were removed by lyophilization and the dry lipid film was resuspended in 1 ml dye buffer solution (70 mM calcein, 10 mM Tris-HCl, 0.1 mM EDTA, 150 mM NaCl, pH 7.4) by gently vortexing. Liposomes were prepared through 10 freeze-thaw cycles in liquid nitrogen followed by an incubation in a 50°C water bath. The suspensions were then extruded 20 times through 100-nm pore-polycarbonate membranes. After extrusion, the resulting calcein-entrapped large unilamellar vesicles (LUVs) were separated from the entrapped calcein using gel filtration through a Sephadex G-50 column, and eluted with buffer (10 mM Tris-HCl, 0.1 mM EDTA, 150 mM NaCl, pH 7.4). Calcein leakage from LUVs was measured at 20°C by monitoring the fluorescence intensity at an excitation wavelength of 490 nm and an emission wavelength of 520 nm on a spectrophotometer (RF-5301PC, Shimadzu, Japan). The fluorescence intensities were recorded as a function of time from 0 to 1100S. Complete release of calcein was induced by the addition of 10% Triton X-100.
Membrane depolarization assay
Cytoplasmic membrane depolarization activity of the peptide was measured using the membrane potential-sensitive dye, DiSC(3)−5. S. aureus were grown at 37°C to mid-log phase (OD600 = 0.5) and were harvested by centrifugation. Cells were washed thrice with buffer (5 mM HEPES, 20 mM glucose, pH 7.4) and resuspended to an OD600 of 0.08 in buffer (5 mM HEPES, 20 mM glucose, 10 mM KCl, pH 7.4) containing KCl to equilibrate K+ levels. Membrane depolarization was recorded by measuring changes in the intensity of fluorescence emission after peptide addition. The excitation and emission wavelengths were 620 and 670 nm, respectively. The membrane potential was completely dissipated by adding gramicidin D.
Results and discussion
Phylogenetic analysis of BC34-6 strain
To identify potential antibiotic agents, bacterial strains were first isolated from red clay soil. A total of 165 samples were collected, from which strain BC34-6 was found to have strong antibacterial activity against E. coli. The phylogenetic tree for strain BC34-6 is shown in Fig. 3, which was constructed based on the rate of nucleotide substitution and indicates the BC34-6 strain belongs to the genus Paenibacillus. The 16S rRNA sequence of this strain was most similar to P. elgii SD17 (GenBank: AY090110, 99%), suggesting the BC34-6 strain belongs to the species P. elgii.
Identification and purification of PGP-E
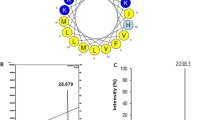
Antimicrobial substances in the culture broth of BC34-6 were initially purified using Diaion HP-20 chromatography. Because Diaion HP-20 resin is an effective absorbent with extremely large pores, Diaion HP-20 chromatography is useful for the purification of peptides, proteins, and fermentation products. The 80% ethanol elution fraction displayed high antimicrobial activity and was concentrated by vacuum evaporation for further purification of antimicrobial substances. We obtained 0.25 g l−1 of crude product from the initial purification. The 80% ethanol fraction contained substances with masses ( = [M + H]+) of 1073.3, 1101.7, 1087.3, and 1088.0 Da, which correspond to the masses of PGP-A, -B, -C, and -D, respectively (Supplementary Figure S1). This strongly indicates that this fraction contains members of the PGP family. From analysis of culture products by liquid chromatography-mass spectrometry, we identified a new peak with strong antimicrobial activity and a different retention time from (Fig. 4a), but same molecular mass as PGP-A (Fig. 4b). Therefore, we hypothesized that this new peak belonged to a new PGP peptide analog, which was named PGP-E. We purified PGP-E using preparative RP-HPLC, which yielded 1.5 mg per 1-liter culture (Fig. 4c).
Reversed-phase high-performance liquid chromatography (RP-HPLC) and liquid chromatography-mass spectrometry (LC-MS) analysis of PGP-E a Chromatogram from RP-HPLC of the 80% ethanol fraction of Diaion HP-20. This fraction contained PGP peptides. PGP-E is indicated in bold. b Mass spectrum of purified PGP-E by LC-MS analysis. c Chromatogram from analytical RP-HPLC of purified PGP-E
Structural elucidation of PGP-E
PGP-E had an [M + H]+ ion peak at m/z 1073.3 and an [M + 2 H]2+ ion peak at m/z 537.3 (Fig. 4b). To determine the amino acid sequence of PGP-E, ESI-MS/MS was used to analyze lactone ring-opened PGP-E, due to the difficulty of annotating cyclic peptides using MS/MS spectra. The doubly charged ion [M + 2 H]2+ was chosen as a precursor ion for further MS/MS fragmentation. The fragments mainly contained b- and y-type ions. Successive fragmentation of the two termini of ring-opened PGP-E resulted in b-type ions at m/z 873.56, 774.49, 674.42, 561.34, 414.27, 314.21, and 215.14 along with corresponding y-type ions detected at m/z 877.55, 778.48, 678.42, 531.35, 418.27, 318.20, and 219.13 (Fig. 5). These fragment ions revealed that PGP-E consists of the following amino acid sequence: Dab1, Val2, Dab3, Phe4, Leu5, Dab6, Val7, and Leu8. As a result, PGP-E has the same amino acid sequence as PGP-A and -C, but different fatty acid moieties. The complete molecular structure of PGP-E was determined using 2D NMR experiments. The 1H and 13C NMR spectra PGP-E assignments are listed in Table 1. The 1H NMR spectrum contained 9 N-bonded protons (δ 7.79–8.60) and 9 α-protons (δ 4.07–4.58) from peptide bonds and one benzene ring of Phe4 (δ 7.27–7.34). The 13C NMR spectrum revealed the presence of 8 carbonyl groups (δ 172.61–176.13), α-carbons of 9 amino acids (δ 52.94–62.01), and one benzene ring of Phe4 (δ 140.60, 132.13, 131.51, and 129.65; 4 signals due to symmetry). Nine amino acids, 3 Dab, 2 Val, 2 Leu, 1 Phe, and 1 Ser, were identified based on TOCSY, DQF-COSY, NOESY, HSQC, and HMBC experiments (Supplementary Figure S2-S8). The sequence of the amino acid residues was confirmed using NOESY (Fig. 6a) with the complete amino acid sequence containing a straight lipid chain (–CH2CH2CH3). This chain was confirmed based on HMBCs between H2′ and C1′/C3′, H3′ and C1′, H4′ and C3′/C5′/C6′, and H5′ and C3′/C4′/C6′ (Fig. 6b). In summary, PGP-E is a new compound belonging to the polypeptin family (Fig. 6c). The polypeptins are nonadepsipeptides which contain 3 dispersed Dab residues as the cationic portion and two D-amino acids (Phe at position 4 and Val at position 7) among 5 hydrophobic residues. In addition, Val or Ile is in position 2 and Ser or Thr is in position 9. The polypeptins also vary at the branched β-hydroxyalkanoic acid residue, whose hydroxyl moiety is linked to the C-terminal residue by an ester bond [18]. The stereochemistry at the 3-hydroxy position of the 2-methylpentanoate was confirmed by 1D 1H and 2D [15N,1HN]-SOFAST-HMQC spectra to be the pro-S configuration. To date, four PGP derivatives have been reported and the total stereochemistry of PGPs is as follows: L-Dab, L-Val/L-Ile, L-Dab, D-Phe, L-Leu, L-Dab, D-Val, L-Leu, and L-Ser [16, 17]. Although the stereochemistry of amino acids in PGP-E has not been experimentally determined in this study, it has been shown that the newly identified PGP-E belonging to the polypeptin family has the same amino acid composition and sequence as PGP-A and -C. Moreover, the TOCSY NMR spectra of PGP-A and PGP-E are very similar except for fatty acid chains. (Supplementary Figure S9). These results suggest that PGP-E has the same stereochemistry of amino acid and ester bond as PGP-A and -C.
NMR spectra and PGP-E structure. a Sequential dαN(i, i + 1) NOE connectivities for PGP-E determined using 1H 2D NOESY in DMSO-d6. Intra-residue NH-CαH cross peaks are labeled with the PGP-E residue number. Right NMR spectrum indicates the lipid chain region of PGP-E. b Heteronuclear multiple-bond correlation (HMBC) spectrum of lipid chain of PGP-E. c Molecular structure for PGP-E. Bold lines indicate different structural regions among the PGP family
Antimicrobial activity of PGP-E
The antimicrobial activity of the newly identified PGP-E was examined by determining the MICs against gram-negative, gram-positive, and MRSA bacteria. PGP-E displayed strong antimicrobial activity with MICs of 2–8 µg ml−1 (Table 2). Other previously reported PGP derivatives (PGP-A, B, C, and D) also showed similar antimicrobial activity in the range of 1–8 µg ml−1 for all tested strains (data not shown). In addition, to compare the antimicrobial activity of PGP-E to similar CLPs, we purified the well-studied CLPs bacillomycin D and surfactin from the culture media of Bacillus velezensis GH1-13. These CLPs exhibited much less antimicrobial activity than that of PGP-E, with MICs of 25–100 µg ml−1 against gram-negative and -positive bacteria. Despite detailed experimental studies, these results suggest PGP-E had more effective antimicrobial activity than Bacillus CLPs. Furthermore, PGP-E had an MIC of 8 µg ml−1 against MRSA strains. These results suggest PGP-E has strong potential as an antimicrobial agent against various pathogenic bacteria.
Hemolytic activity of PGP-E
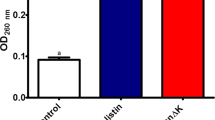
The cytotoxicity of PGP-E was analyzed based on its ability to lyse HRBCs. PGP-E caused hemolysis in less than 50% of cells, at 200 µM (Fig. 7a), a concentration that effectively inhibited growth of all tested bacteria. Melittin, which was used as a control, caused hemolysis in almost 90% of cells at 25 µM.
a Hemolytic activity of PGP-E against human red blood cells. b Time-dependent peptide-induced calcein release from calcein-entrapped negatively charged EYPE/EYPG (7:3, w/w) large unilamellar vesicles by PGP-E (8 µg ml−1), melittin (32 µg ml−1), buforin-2 (16 µg ml−1), and LL-37 (4 µg ml−1). c Time-dependent membrane depolarization of S. aureus by PGP-E (8 µg ml−1), melittin (16 µg ml−1), buforin-2 (16 µg ml−1), and LL-37 (4 µg ml−1)
Mechanism of action
Antimicrobial peptides composed of cationic and hydrophobic residues are well suited for interacting with cell membranes and perturbing membrane structures. Similarly, PGP-E is a cationic CLP containing three positively charged Dab residues and five hydrophobic amino acids. To examine the bactericidal mechanism of PGP-E, leakage of fluorescent dye calcein-entrapped LUVs composed of negatively charged EYPE/EYPG (7:3, w/w) and depolarization of bacterial cytoplasmic membranes using a membrane potential-sensitive dye, DiSC3-(5) were employed. LL-37 and melittin, membrane-targeting (pore-forming/disrupting) peptides, were used as positive controls. Buforin-2, an intracellular-targeting (non-membrane-targeting) peptide, was used as a negative control. PGP-E induced calcein dye leakage from negatively charged EYPE/EYPG (7:3, w/w) LUVs in a time-dependent (Fig. 7b) manner. PGP-E (8 µg ml−1) and LL-37 (4 µg ml−1) induced dye leakage of 63 and 93%, respectively, from EYPE/EYPG (7:3, w/w) LUVs within 1000 s. Melittin induced 100% leakage within a few seconds at a concentration of 16 µg ml−1. Next, the ability of PGP-E to permeabilize the membranes of intact S. aureus cells was assessed. Due to cell membrane potential, the dye DiSC3-(5) can concentrate in the cytoplasmic membrane, resulting in self-quenching of fluorescence. Upon disruption of the membrane potential, the dye is released into the buffer, causing an increase in fluorescence intensity. In the present study, the fluorescence intensity of DiSC3-(5) was quenched because the dye accumulated in the membrane (Fig. 7c). Peptides were added after the signal was stable for 400 s (arrow in Fig. 7c). It was found PGP-E depolarized the bacterial cytoplasmic membrane in a time-dependent manner. Similar to LL-37, the addition of PGP-E caused a gradual increase in fluorescence intensity due to a collapse of the ion gradients generating the membrane potential. Melittin (16 µg ml−1) induced complete depolarization of the cytoplasmic membranes of S. aureus within a few seconds. However, buforin-2 did not induce any dye leakage or membrane depolarization. The bactericidal mechanism of antimicrobial peptides typically involves destroying microbial membranes or targeting intracellular components. PGP-E induced dye leakage from bacterial membrane-mimicking EYPE/EYPG (7:3, w/w) LUVs and caused membrane depolarization in intact S. aureus cells. The loss of membrane potential in S. aureus cells due to PGP-E was comparable to that caused by the lytic gramicidin D, a peptide that can form transmembrane pores and ionic channels [19]. In addition, the gradual increase in dye release and loss of membrane potential observed suggest PGP-E is bactericidal due to its ability to damage membrane integrity [20].
Significance of PGP-E as an antimicrobial agent
The constantly growing drug resistance of bacteria has triggered an urgent need to identify alternative antimicrobial agents, such as lipopeptides, for use in dairy products as well as clinical applications [6]. Lipopeptides are powerful multipurpose materials used for dealing with various pathogens. Microorganisms of the Bacillus genus are considered efficient microbial factories for large-scale production of these lipopeptides [21, 22]. The Bacillus lipopeptide families, including surfactin, iturin, and fengycin, have been studied due to their potent antagonistic activity against various phytopathogens, including bacteria, fungi, and oomycetes [23]. Among these, the surfactin produced by B. subtilis is a major representative of the lipopeptide family. Surfactin has excellent membrane-active and surface-interfacial properties that instill important biological activity. Because of this nature, surfactin is a strong potential candidate for use in medicine and environmental protection [24]. Surfactin has bacteriostatic and bactericidal properties for gram-negative and -positive bacteria, and can potentially be used in commercial antibiotic preparations for medical and pharmaceutical applications. However, the critical disadvantage of surfactin is its nonspecific cytotoxicity [25,26,27]. This is because surfactin can lyse not only animal cells, but also pathogenic cells. Attention must be paid during intravenous administration as surfactin has the ability to lyse red blood cells. The newly isolated PGP-E is a lipopeptide with strong antibacterial activity with MICs of 2–8 μg ml−1 of MIC for various bacteria, including MRSA strains, but low hemolytic activity. Although further in vitro and in vivo studies for PGP-E are needed to evaluate its biological activity and cytotoxicity, PGP-E is a new candidate antimicrobial agent that may be highly applicable in various applications, including human healthcare, industry, agriculture, and environmental protection.
References
Frieri M, Kumar K, Boutin A. Antibiotic resistance. J Infect Public Health. 2017;10:369–78.
Ribeiro SM, et al. New frontiers for anti-biofilm drug development. Pharmacol Ther. 2016;160:133–44.
Mnif I, Ghribi D. Review lipopeptides biosurfactants: mean classes and new insights for industrial, biomedical, and environmental applications. Biopolymers. 2015;104:129–47.
Muto CA, et al. SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and enterococcus. Infect Control Hosp Epidemiol. 2003;24:362–86.
Schneider T, Muller A, Miess H, Gross H. Cyclic lipopeptides as antibacterial agents—potent antibiotic activity mediated by intriguing mode of actions. Int J Med Microbiol. 2014;304:37–43.
Mandal SM, Sharma S, Pinnaka AK, Kumari A, Korpole S. Isolation and characterization of diverse antimicrobial lipopeptides produced by Citrobacter and Enterobacter. BMC Microbiol. 2013;13:152.
Ines M, Dhouha G. Lipopeptide surfactants: production, recovery and pore forming capacity. Peptides. 2015;71:100–12.
Klevens RM, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–71.
Sharma D, Mandal SM, Manhas RK. Purification and characterization of a novel lipopeptide from Streptomyces amritsarensis sp. nov. active against methicillin-resistant Staphylococcus aureus. AMB Express. 2014;4:50.
Cochrane SA, Vederas JC. Lipopeptides from Bacillus and Paenibacillus spp.: a gold mine of antibiotic candidates. Med Res Rev. 2016;36:4–31.
Ash C, Priest FG, Collins MD. 3 bacilli (Ash, Farrow, Wallbanks and Collins) using a PCR probe test. Proposal for the creation of a new genus. Paenibacillus Antonie Van Leeuwenhoek. 1993;64:253–60. Molecular identification of rRNA group
Montes MJ, Mercade E, Bozal N, Guinea J. Paenibacillus antarcticus sp. nov., a novel psychrotolerant organism from the Antarctic environment. Int J Syst Evol Microbiol. 2004;54(Pt 5):1521–6.
Saha P, Mondal AK, Mayilraj S, Krishnamurthi S, Bhattacharya A, Chakrabarti T. Paenibacillus assamensis sp. nov., a novel bacterium isolated from a warm spring in Assam, India. Int J Syst Evol Microbiol. 2005;55(Pt 6):2577–81.
Sanchez MM, et al. Paenibacillus barcinonensis sp. nov., a xylanase-producing bacterium isolated from a rice field in the Ebro River delta. Int J Syst Evol Microbiol. 2005;55(Pt 2):935–9.
Yoon JH, Kang SJ, Yeo SH, Oh TK. Paenibacillus alkaliterrae sp. nov., isolated from an alkaline soil in Korea. Int J Syst Evol Microbiol. 2005;55(Pt 6):2339–44.
Wu XC, Shen XB, Ding R, Qian CD, Fang HH, Li O. Isolation and partial characterization of antibiotics produced by Paenibacillus elgii B69. FEMS Microbiol Lett. 2010;310:32–38.
Ding R, et al. Isolation and identification of lipopeptide antibiotics from Paenibacillus elgii B69 with inhibitory activity against methicillin-resistant Staphylococcus aureus. J Microbiol. 2011;49:942–9.
Mountford SJ, et al. The first total synthesis and solution structure of a polypeptin, PE2, a cyclic lipopeptide with broad spectrum antibiotic activity. Org Biomol Chem. 2017;15:7173–80.
Burkhart BM, Gassman RM, Langs DA, Pangborn WA, Duax WL. Heterodimer formation and crystal nucleation of gramicidin D. Biophys J. 1998;75:2135–46.
Manzini MC, et al. Peptide: lipid ratio and membrane surface charge determine the mechanism of action of the antimicrobial peptide BP100. Conformational and functional studies. Biochim Biophys Acta. 2014;1838:1985–99.
Emmert EA, Handelsman J. Biocontrol of plant disease: a (gram-) positive perspective. FEMS Microbiol Lett. 1999;171:1–9.
Roongsawang N, Washio K, Morikawa M. Diversity of nonribosomal peptide synthetases involved in the biosynthesis of lipopeptide biosurfactants. Int J Mol Sci. 2011;12:141–72.
Ongena M, Jacques P. Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol. 2008;16:115–25.
Meena KR, Kanwar SS. Lipopeptides as the antifungal and antibacterial agents: applications in food safety and therapeutics. Biomed Res Int. 2015;2015:473050.
Heerklotz H, Seelig J. Detergent-like action of the antibiotic peptide surfactin on lipid membranes. Biophys J. 2001;81:1547–54.
Dufour S, Deleu M, Nott K, Wathelet B, Thonart P, Paquot M. Hemolytic activity of new linear surfactin analogs in relation to their physico-chemical properties. Biochim Biophys Acta. 2005;1726:87–95.
Kragh-Hansen U, le Maire M, Moller JV. The mechanism of detergent solubilization of liposomes and protein-containing membranes. Biophys J. 1998;75:2932–46.
Acknowledgements
This study was supported by the Bio-industry Technology Development Program 2012, Ministry for Food, Agriculture, Forestry and Fisheries, (Grant No. 112003-3) and “Cooperative Research Program for Agricultural Science & Technology Development (Project No. PJ012467)”, Rural Development Administration, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Kim, J., Il Kim, P., Bong, K.M. et al. Isolation and structural elucidation of pelgipeptin E, a novel pore-forming pelgipeptin analog from Paenibacillus elgii with low hemolytic activity. J Antibiot 71, 1008–1017 (2018). https://doi.org/10.1038/s41429-018-0095-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-018-0095-2
This article is cited by
-
Complete genome sequence of biocontrol strain Paenibacillus peoriae HJ-2 and further analysis of its biocontrol mechanism
BMC Genomics (2022)
-
A novel family of non-secreted tridecaptin lipopeptide produced by Paenibacillus elgii
Amino Acids (2022)
-
Co-occurrence of linear and cyclic pelgipeptins in broth cultures of Paenibacillus elgii AC13
Brazilian Journal of Microbiology (2021)
-
Streptomyces sp SM01 isolated from Indian soil produces a novel antibiotic picolinamycin effective against multi drug resistant bacterial strains
Scientific Reports (2020)