Abstract
Legionella pneumophila is a waterborne intracellular pathogenic bacterium, the most frequent cause of human legionellosis and a relatively common cause of community-acquired and nosocomial pneumonia. Some legionellosis outbreaks are related to the presence of biofilms, which provide a reservoir for L. pneumophila strains. We investigated the in vitro activities of antibiotics; erythromycin and doxycycline, antimicrobial peptides AMPs; melittin, LL-37 and CAMA (cecropin A (1–7)—Melittin A (2–9) and ceragenins; CSA-8, CSA-13, CSA-44, CSA-131 and CSA-138 against L. pneumophila. Isolation of Legionella strains was conducted according to ISO 1998. Minimum inhibitory concentrations (MICs), minimum bactericidal concentrations (MBCs) and minimum biofilm eradication concentrations (MBECs) were determined using microbroth dilution techniques. MIC ranges for melittin, LL-37, and CAMA were 0.25–1, 1–4, and 2–8 µg ml−1, respectively. MIC ranges for CSA-8, 13, 44, 131, and 138 were 0.5–2, 0.5–1, 1–4, 0.5–2, and 1–2 µg ml−1, respectively, and MBEC values for the ceragenins were 10–160 µg ml−1. These results demonstrate that AMPs and ceragenins display broad-spectrum, in vitro activity against L. pneumophila. In particular, CSA-8, CSA-13 and melittin gave the lowest MICs and MBCs. We also observed that ceragenins are active against established L. pneumophila biofilms.
Similar content being viewed by others
Introduction
Legionella pneumophila is an intracellular pathogen which can cause one of two forms of disease; legionnaires’ disease (LD), which in the pneumonal form can be fatal, and Pontiac fever, a milder form of the disease similar to influenza in humans [1, 2]. To date, more than 54 species of Legionella have been identified, L. pneumophila is associated with 91.5% of reported cases worldwide with L. pneumophila serogroup 1 causing 84.2% of these cases [3]. In contrast, L. pneumophila sg 2–14 account for only 15–20% of community-acquired cases, although they account for over 50% of the isolates obtained from man-made water systems [4]. L. pneumophila has been increasingly recognized as a significant cause of sporadic and epidemic community-acquired pneumonia (CAP) and nosocomial pneumonia in both healthy and immuno-suppressed hosts [5, 6]. L. pneumophila is ubiquitously found in natural fresh water and man-made water systems. These systems are mainly responsible for outbreaks as they can produce contaminated water droplets, which are inhaled. This is especially true with association with biofilms [7]; some legionellosis outbreaks are related to the presence of biofilms, a major reservoir for L. pneumophila [8].
Biofilms are populations of cells often growing on surfaces and embedded in an extracellular polymeric substance (EPS) [9, 10]. Biofilms may form on living or non-living surfaces and can be prevalent in natural, industrial, and hospital settings. Biofilms pose a serious problem for public health because of the increased resistance of biofilm-associated organisms to antimicrobial agents and the potential for these organisms to cause infections in patients [11, 12].
Antimicrobial peptides (AMPs) are a major component of the innate immune system in higher organisms and play an important role in host defense in organisms ranging from insects to plants to mammals. AMPs have rapid action and a broad spectrum of activity against Gram-negative and positive bacteria, fungi, viruses, and parasites. But, many AMPs are difficult to synthesize and purify due to their complexity and size [13, 14].
Ceragenins were developed as non-peptide mimics of AMPs and display properties that may make them useful antimicrobial agents. These compounds have advantages over AMPs in that they are resistant to proteolysis, they have mild toxicity and they are relatively simple to prepare and purify on a large scale. Like AMPs, ceragenins exhibit rapid bactericidal activity against a broad range of bacterial species [15,16,17]. As well as their antibacterial activities, these molecules also show antifungal, antiviral, antiparasitic, antibiofilm, and anticancer effects [18, 19].
In general, however L. pneumophila is a antibiotic sensitive and easy to treating bacterium, there are limited antibiotics such as quinolone or macrolides, could act against Legionella infections due to its’ intracellular characteristics. However, there is no published data describing the in vitro activities of ceragenins against intracellular pathogens such as L. pneumophila, to our knowledge, only a few AMPs which are different from ours, were evaluated in a limited study [20, 21]. In this study, we aimed to investigate the activities of AMPs and ceragenins against L. pneumophila, an important intracellular pathogen.
For this purpose, we isolated the L. pneumophila strains from water systems with our in-hause procedure, then we tested the antibacterial activities of some AMPs, ceragenins and antibiotics against them. We also investigated the anti biofilm activities of ceragenins which are more possible candidate as new antimicrobial group due to their advantages, against biofilm forms of L. pneumophila isolates.
Materials and methods
Bacterial strains
Twenty L. pneumophila strains used for this study were isolated from hot and cold water systems of different hotels, geographically located in close proximity in Istanbul, Turkey. L. pneumophila ATCC 33152 (American Type Culture Collection, Rockville, Md. USA) was used as a quality control strain. Staphylococcus aureus ATCC 29213 was used to verify the accuracy of the microdilution test procedure for antibiotics.
Isolation of L. pneumophila strains
Isolation of Legionella strains was conducted according to ISO 11731 (1998). Water samples (3 l) were concentrated with filtration, 2 ml of concentrated samples were incubated at 50 °C for 30 min, and 10 ml from each sample was treated with 1:1 HCl-KCl (pH:2.2). Each of these processed water samples (0.1 ml) were streaked onto buffered charcoal yeast extract agar (BCYE) supplemented with glycine, vancomycin, polymyxin and cycloheximide. All plates were incubated at 37 °C for 10–14 days. Colonies consistent with Legionella morphology were subcultured on tryptone soy agar. Analysis of these strains was conducted according to ISO 11731 (1998). Serotyping was performed with a latex agglutination test kit (Oxoid) [22]. Additionally, we investigated genotypic features of L. pneumophila serogroup 1 and serogroup 2–14 strains isolated by randomly amplified polymorphic DNA (RAPD) method [23].
Antimicrobial agents
AMPs; melittin, LL-37 and CAMA (cecropin A (1-7) – Melittin A (2-9) were obtained from Bachem AG (Bubendorf, Switzerland). Ceragenins; CSA-8, CSA-13, CSA-44, CSA-131 and CSA-138 (Fig. 1) were synthesized from a cholic acid scaffold as previously described [24]. Comparator antibiotics; erythromycin and doxycycline were kindly provided from respective manufacturers. Stock solutions from dry powders were prepared according to the manufacturers’ recommendation and stored frozen at −80 °C for up to 6 months.
Media
Buffered yeast extract (BYE) broth was used for minimum inhibitory concentration (MIC) and minimum biofilm eradication concentration (MBEC) measurements. BYE broth was supplemented with l-cystein and ferric pyrophosphate [25]. Plates of buffered BCYE agar were used for minimum bactericidal concentration (MBC) and MBEC determinations and colony counts.
MIC and MBC determinations
MICs of AMPs, ceragenins, and antibiotics were determined by the microbroth dilution technique described by the Clinical and Laboratory Standards Institute (CLSI) [26, 27]. Serial two-fold dilutions ranging from 128 to 0.125 µg ml−1 and 16 to 0.0156 µg ml−1 were prepared in BYE broth for ceragenins, AMPs and erythromycin, doxycycline, respectively. A suspension of each strain was prepared in BYE broth. The turbidity of the broth culture was adjusted to an optical density equivalent to 0.5 McFarland standards, diluted in BYE broth to give a final concentration of 5 × 105 cfu ml−1 on the test plates. Plates were covered and placed in plastic bags to prevent evaporation and incubated at 35 oC for 48 h. The MIC was defined as the lowest concentration of antibiotic giving complete inhibition of visible growth.
MBCs were determined at the end of the incubation period by removing two 10 µl samples from each well demonstrating no visible growth and plating onto BCYE agar. Resultant colonies were counted after incubation for 48 h at 37 °C. The MBC was defined as the lowest concentration of antimicrobials producing at least 99.9% (three-log) killing of the initial inocula [28].
Biofilm formation
Biofilm formation was assayed using cells grown on BCYE agar plates at 37 °C, 5% CO2 for 3-4 days. BYE medium was used to grow L. pneumophila planktonically and monospecies biofilms. Cells were resuspended in BYE at OD625 0.20 and diluted to give a final concentration of approximately 1 × 107 cfu/500 µl. This suspension was added to the wells of 24-well tissue culture microtitre plates (Greiner) and incubated at 37 °C for seven days. The negative control was prepared in BYE broth. After the incubation, waste media was aspirated gently and the wells of the plates were washed two times with 500 µl BYE broth to remove unattached bacteria and air-dried. 500 µl of 99% methanol was added per well for 15 min for fixation and aspirated, and plates were allowed to dry. Wells were stained with 500 μl of 0.1% crystal violet (in water) for 5 min. Excess stain was gently rinsed off with water, and plates were air-dried. Stain was resolubilized in 500 μl of 95% ethanol, shaking in orbital shaker for 30 min. and measured at OD595 nm [29].
Minimum biofilm eradication concentration (MBEC)
MBEC values for the ceragenins and antibiotics were determined. Five biofilm producing L. pneumophila isolates were used for MBEC determinations. Measurements of the antimicrobial susceptibilities of L. pneumophila biofilms were performed as previously described for the MBEC assay with the following modifications [29, 30]. The biofilms (seven-day), grown in a 24-well tissue culture microtitre plates, were washed three times with 500 µl BYE solutions and air-dried. Serial, two-fold dilutions ranging from 320 to 10 µg ml−1 for ceragenins, 1280 to 40 µg ml−1 for erithromycin and doxycyline were prepared in BYE broth. Aliquots (500 µl) of solution at each concentration were added to each corresponding well and plates were incubated for 48 h at 37 °C. After incubation, ceregenins and antibiotics were aspirated gently and plates were washed two times with BYE broth, wells were scraped throughly, with particular attention to well edges. Well contents were removed, placed in BYE broth (1 ml), sonicated in a sonicating waterbath (Bandelin sonopuls HD 2200) for 5 min to disrupt the biofilm, and 100 µl samples were plated on BCYE agar. Colonies were counted after 48 h at 37 °C. The MBEC was defined as the lowest concentration of antimicrobials that prevented bacterial regrowth.
Results
Isolation of L. pneumophila strains
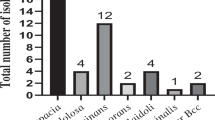
Eight of the 20 L. pneumophila strains isolated were identified as L. pneumophila serogroup 1 and 12 were identified as L. pneumophila serogroups 2–14. Regions where L. pneumophila strains were isolated on the plumbing system were cooling tower (two isolates), cool water storage (one isolate), hot water storage (seven isolates), hot water tap (six isolates) and hot water shower head (four isolates) (Fig. 2).
MIC and MBC determinations
The in vitro activities of AMPs, ceragenins and conventional antibiotics against 20 L. pneumophila strains are summarized in Table 1.
Susceptibility testing demonstrated that the MIC ranges for CSAs-8, 13, 44, 131, and 138 were 0.5–2, 0.5–1, 1–4, 0.5–2, and 1–2 µg ml−1 and MBC ranges for those were 0.5–4, 1–2, 1–8, 0.5–2, and 2 µg ml−1, respectively. The MIC ranges for melittin, LL-37 and CM were 0.25–1, 1–4, and 2–8 µg ml−1 and MBC ranges for those were 0.5–2, 2–16, and 2–16 µg ml−1. There was a slight difference between MICs of serogroup 1 and serogroup 2–14 strains. As seen from the results, CSA-13 and melittin showed the lowest pattern of MIC and MBC ranges, for which MIC values varried in a 2–3 dilution range. There was no major difference between bactericidal and inhibitory endpoints. The MBCs were generally equal to or two fold greater than the MICs. On the other hand, CSA-8, CSA-13, and melittin showed almost the same activity as doxcycline.
With the control strain L. pneumophila ATCC 33152, CSA-8, CSA-13, CSA-44, CSA-131, and CSA-138 MICs and MBCs were 2, 1, 4, 2, 2 and 4, 2, 4, 2, 2 µg ml−1, respectively. The MIC ranges for melittin, LL-37 and CM were 0.5, 2, 2 and MBC ranges for those were 1, 2, 4 µg ml−1. Since there are no data about the breakpoints of L. pneumophila ATCC 33152 in the CLSI, we used S. aureus ATCC 29213 for the standardization of the experiments. The MIC values of the antibiotics against the quality control strain S. aureus ATCC 29213 were within the accuracy range in CLSI throughout the study [27].
Minimum biofilm eradication concentration (MBEC)
The anti-biofilm activities of five ceragenins, erythromycin and doxycycline against L. pneumophia isolates are summarized in Table 2. MBEC values of ceragenins and antibiotics ranged between 10–320 µg ml−1.
Discussion
Hotels are important locations for infections caused by Legionella species. In our study, water samples taken from hot and cold water systems of different hotels located in geography of Istanbul were investigated. 40% of the twenty L. pneumophila strains isolated from these water systems were identified as L. pneumophila serogroup 1, 60% L. pneumophila serogroups 2–14. These findings suggest that serogroup 2–14 is often isolated from water systems, although it has a low pathogenicity compared to serogroup 1. Also, 85% of L. pneumophila strains were isolated from hot water systems similar to those obtained by other researchers [31, 32].
Serious infections caused by L. pneumophila still remain an important public health problem in the world. Mortality is high for those with nosocomial pneumonia, especially immunocompromised and bacteremic patients [33]. It is widely known that macrolides, fluoroquinolones, rifampicin, doxycycline or trimethoprim-sulfamethoxazole are effective for these kind of infections [34, 35]. However, comprehensive clinical trials to determine the incidence and case mortality rates of their use are scarce. In addition, variations in the incidence of Legionnaires’ disease indicate that new knowledge is needed in order to better understand the burden of disease and determine the best forms of treatment [36, 37]. As a Gram-negative bacterium, L. pneumophilia is inherently resistant to many antibiotics due to the permeability barrier provided by the outer membrane, and emergence of multi-drug resistant forms of this pathogen [38] further underscores the need for new therapeutics. As mimics of endogenous AMPs the ceragenins offer a mechanism of action distinct from most other antibiotics. By selectively targeting bacterial membranes, bacteria are not readily able to develop high levels of resistance.
In this study, we investigated the in vitro activities of erythromycin and doxycyclin, three AMPs and five ceragenins against L. pneumophila strains. When we examined the MIC values of antibiotics against L. pneumophila strains, the MIC50 and MIC90 values for erithromycin (0.25 µg ml−1,) and doxycycline (1–2 µg ml−1) were similar or higher compared to those previously reported [18, 39]. Also, the MIC values of AMPs were within the 0.5–2 µg ml−1, range. Melittin was found to be the most effective cationic peptide, when MBC50 and MBC90 values were considered (1 µg ml−1). Cecropin-melittin is hybrid peptide that contain portions of the amino acid sequences of cecropin A and melittin. The hybrid peptide has demonstrated improved antimicrobial activity against Gram-positive bacteria with a significant reduction of the toxicity associated with melittin [40]. This hybrid peptide and LL37 (cathelicidin-derived AMP found in humans) were found have in vitro activity against L. pneumophila. MIC50/MBC50 ratios of the studied AMPs were 0.5/1 µg ml−1 for melittin, 2/2 µg ml−1 for LL37, and 2/4 µg ml−1 for CM.
Ceragenins include compounds with potent bactericidal activities against both Gram-negative and Gram-positive bacteria. CSA-8 is somewhat less active against Gram-negative than other ceragenins [15,16,17]. CSA-44 is a degradable form of ceragenin with a half life in aqueous solution at pH 7 of 37 days. When it degrades, it returns to cholic acid (a common bile acid), beta alanine (an endogenous amino acid), and octanol (a waxy alcohol). For many applications CSA-44 appears well suited because it is easy to prepare on a large scale and its degradation products are well characterized. CSAs 131 and 138 are closely related to CSA-13. The only difference is that the lipid chain extending from the amine at C24 is longer in these two compounds as compared to CSA-13. The lipid chain lengths are 8, 12, and 13 carbons for CSA-13, -131, and -138, respectively. The longer carbon chains are intended to allow stronger association with membrane components of Gram-negative bacteria.
Susceptibility data from our results demonstrated that the MIC90 values of the five ceragenins were ranked as follows: CSA-13 < 8 < 131 = 138 < 44 indicating that CSA-13 and CSA-8 were the most active agents in both serogroup 1 and serogroup 2–14 (Table 1). Although CSA-8 is generally more potent against Gram-positive bacteria [40], here we obtained low MICs of CSA-8 against L. pneumophila strains. All studied ceragenins (CSA-8, 13, 44, 131, and 138) against L. pneumophila serogroup 2–14 have an MIC50/MBC50 ratio of 1, suggesting bactericidal activity. Ceragenins CSA-13 and CSA-8 have potent in vitro activities against L. pneumophila with MIC90 of 1 µg ml−1, which is comparable to that of doxycyline and slightly less active than erithromycin, which has an MIC90 value of 0.25 µg ml−1. The MBC50 values of ceragenins were the same or lower than the MBC50 of erithromycin and doxycycline against serogroup 1 of L. pneumophila strains.
Isolation of L. pneumophila from hot water systems demonstrates that the organisms grow at high temperatures. L. pneumophila, in contrast to other Gram-negative bacteria, have branched fatty acid chains and a less dense group of esters in their membrane components. In fact, there are similarities between the membrane structure of L. pneumophila and thermophylic bacteria. Many agents that affect bacterial membranes are cationic and superficially amphiphilic, including AMPs or ceragenins [41]. AMPs and ceragenins are found to have potent bactericidal activity against both Gram-negative and Gram-positive bacteria, and their most frequently studied targets is the bacterial membrane. According to our results, studied AMPs and ceragenins have potent antibacterial activities against L. pneumophila strains. This results suggested that, those antimicrobials are easily get into the cell and act against the intracellular bacterial membranes such as L. pneumophila.
In a present study, we also investigated the anti-biofilm activities of ceragenins comparatively with erythromycin and doxycyclin, and MBECs were found in the range of 10–160 µg ml−1 with ceragenins and 20–320 µg ml−1 with erythromycin and doxycyclin. The MBECs were higher than the MICs for all isolates as expected. MBEC/MIC ratios of these antibiotics and ceragenins were found between 80–320 and 20–80 fold, respectively. In the treatment of infections associated with L. pneumophila biofilm, it has also verified that, determination of MIC value is not always sufficient, and higher doses of antimicrobials are required. Commonly, the MBEC/MIC ratios of antibiotics are up to 1000 fold, and that high doses are beyond the non-toxic therapeudic doses. Therefore, the MBEC/MIC ratio is very important in the treatment of infections caused by biofilm-forming isolates. According to our results, erythromycin, doxycycline and five ceragenins were active against L. pneumophila biofilms, and their MBEC/MIC ratios seems to be in acceptable values. To the extent that infections with L. pneumophilia include a biofilm component, ceragenins may offer a means of eliminating infections that are resistant to other antibiotics.
In conclusion, our results demonstrate that ceragenins and AMPs show a broad spectrum of in vitro activity against L. pneumophila. In particular, CSA-8, CSA-13, and melittin were shown to have advantages because they have lower MIC and MBC values. Also, considering the MBEC/MIC ratios of ceragenins, these antimicrobials may be a good option for the treatment of biofilm-associated infections. Because L. pneumophila is an intracellular pathogen, the effectiveness of these new antimicrobial agents in vivo and in vitro cellular infection or animal models should be evaluated.
References
McDade JE, et al. Legionnaires’ disease - isolation of a bacterium and demonstration of its role in other respiratory disease. New Engl J Med. 1977;297:1197–203.
Pasculle W. Update on Legionella. Clin Microbiol Newslett. 2000;22:97–101.
Yu VL, et al. Distribution of Legionella species and serogroups isolated by culture in patients with sporadic community-acquired legionellosis: an international collaborative survey. J Infect Dis. 2002;186:127–8.
Doleans A, et al. Clinical and environmental distributions of Legionella strains in France are different. J Clin Microbiol. 2004;42:458–60.
Carratala J, et al. Outpatient care compared with hospitalization for community-acquired pneumonia: a randomized trial in low-risk patients. Ann Intern Med. 2005;142:165–72.
Levin AS. Nosocomial legionellosis: prevention and management. Expert Rev Anti Infect Ther. 2009;7:57–68.
Keevil CW. Pathogens in environmental biofilms. In: Bitton G, editor. The encyclopedia of environmental microbiology. Morb Mortal Wkly Rep. 2002;49:1–102.
Abdel-Nour M, et al. Biofilms: the stronghold of Legionella pneumophila. Int J Mol Sci. 2013;14:21660e75.
Ceri H, et al. The calgary biofilmdevice: new technology for rapid determination of antibiotic susceptibilities ofbacterial biofilms. J Clin Microbiol. 1999;37:1771–6.
Lewis K. Riddle of biofilm resistance. Antimicrob Agents Chemother. 2001;45:999–1007.
Donlan RM. Biofilm formation: a clinically relevant microbiological process. Clin Infect Dis. 2001;33:1387–92.
Ghannoum M, O’Toole GA. Microbial Biofilms. ASM Press, Washington, DC, 2004.
Döşler S, Karaaslan E, Gerçeker A. Antibacterial and antibiofilm activities of melittin and colistin, alone and in combination with antibiotics against Gram negative bacteria. J Chemother. 2016;28:95–103.
Vanesa CAlbiolMatanic, Castilla. Viviana. Antiviral activity of antimicrobial cationic peptides against Junin virus and herpes simplex virüs. Int J Antimicrob Agents. 2004;23:382–9.
Cagla Bozkurt-Guzel, Paul B. Savage, Akcali A et al. Potential snergy activity of the novel ceragenin, CSA-13, against carbapenem-resistant Acinetobacter baumannii strains isolated from bacteremia patients. BioMed Res International. 2014;710273:1–5.
Chin JN, et al. Potential synergy activity of the novel ceragenin, CSA-13, against clinical isolates of Pseudomonas aeruginosa, including multidrug-resistant P. aeruginosa. J Antimicrob Chemother. 2008;61:365–70.
Chin JN, et al. Antimicrobial activities of ceragenins against clinical isolates of resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2007;51:1268–73.
De Giglio O, et al. Antibiotic susceptibility of Legionella pneumophila strains isolated from hospital water systems in Southern Italy. Environ Res. 2015;142:586–90.
Moscoso M, et al. In vitro bactericidal and bacteriolytic activity of ceragenin CSA13 against planktonic cultures and biofilms of Streptococcus pneumoniae and other pathogenic streptococci. PLoS ONE. 2014;99:1–10.
Marchand A, et al. Anti-Legionella activity of staphylococcal hemolytic peptides. Peptides. 2011;32:845–51.
Schlusselhuber M, et al. Potent antimicrobial peptides against Legionella pneumophila and its environmental host, Acanthamoeba castellanii. Appl Microbiol Biotechnol. 2015;99:4879–91.
British Standard. Microbiological methods, detection and enumeration of Legionella. In: Water Quality part 4. 1998.
Zeybek Z, Türetgen, Kimiran Erdem A, et al. Profiling of environmental Legionella pneumophila strains by randomly amplified polymorphic DNA method isolated from geographically nearby buildings. Environ Monit Assess. 2009;149:323–7.
Qunying G, et al. Preparation and characterization of cholic acid-derived antimicrobial agents with controlled stabilities. Org Lett. 2000;2:2837–40.
Liebers D, et al. Susceptibility of Legionella pneumophila to eight antimicrobial agents including four macrolides under different assay conditions. J Antimicrob Chemother. 1989;23:37–41.
Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 7th edn. Approved standard M7-A7. Clinical and Laboratory Standards Institute, Wayne: PA, USA; 2006.
Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; 2th informational supplement. M100-S21. Clinical and Laboratory Standards Institute, Wayne: PA; 2011.
National Committee for Clinical Laboratory Standards. 1999. Methods for Determining Bactericidal Activity of Antimicrobial Agents: Approved Guideline M26-A. National Committee for Clinical Laboratory Standards, Wayne: PA, USA; 1999.
Hindre´ T, et al. Transcriptional profiling of Legionella pneumophila biofilm cells and the influence of iron on biofilm formation. Microbiology. 2008;154:30–41.
Ceri H, et al. The calgary biofilmdevice: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J Clin Microbiol. 1999;37:1771–6.
Birteksöz AS, Zeybek Z, Çotuk. In vitro activities of various antibiotics against Legionella pneumophila. In: NP Cianciotto et al, editors. Legionella, State Of The Art 30 Years After Its Recognition. 1752 N Street NW, Washington, Legionella; 2006. p. 43–6.
Pringler N, Brydov P, Uldum A. Occurrence of Legionella in Danish water systems. In: Marre R, Kwaik YA, Bartlett C et al, editors. Legionella, ASM, Washington DC, 2002. p.298–301.
Mraz AL, Weir MH. Knowledge to Predict Pathogens: Legionella pneumophila Lifecycle Critical Review Part I Uptake into Host Cells. Water. 2018;10:1–19.
Sabria M, et al. Fluoroquinolones vs macrolides in the treatment of Legionnaires’ disease. Chest. 2005;128:1401–5.
Torre I, et al. Environmental surveillance and in vitro activity of antimicrobial agents against Legionella pneumophila isolated from hospital water systems in Campania, South Italy: a 5-year study. Environ Res. 2018;14:574–79.
Neil K, Berkelman R. Increasing incidence of legionellosis in the United States, 1990-2005: changing epidemiologic trends. Clin Infect Dis. 2008;47:591–99.
Viasus D, et al. Community-acquired Legionella pneumophila Pneumonia A single-center experience with 214 hospitalized sporadic cases over 15 years. Medicine (Baltimore). 2013;92:51–60.
X. Ferhat M, et al. The TolC protein of Legionella pneumophilia plays a major role in multi-drug resistance and the early steps of host invasion. PLoS ONE. 2009;4:e7732.
Bruin JP, et al. Wild-type MIC distribution and epidemiological cut-off values in clinical Legionella pneumophila serogroup 1 isolates. Diagn Microbiol Infect Dis. 2011;72:103–08.
Cao Y, et al. Design, recombinant expression, and antibacterial activity of the cecropins-melittin hybrid antimicrobial peptides. Curr Microbiol. 2010;61:169–75.
Epand RM, Epand RF, Savage PB. Ceragenins (Cationic Steroid Compounds), a novel class of antimicrobial agents. Drug News Perspect. 2008;21:307–11.
Acknowledgements
This work was supported by a grant from the Research Fund of Istanbul University (Project no. 5681 and 42002).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Birteksoz-Tan, A.S., Zeybek, Z., Hacioglu, M. et al. In vitro activities of antimicrobial peptides and ceragenins against Legionella pneumophila. J Antibiot 72, 291–297 (2019). https://doi.org/10.1038/s41429-019-0148-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-019-0148-1
This article is cited by
-
Human Antimicrobial Peptides: Spectrum, Mode of Action and Resistance Mechanisms
International Journal of Peptide Research and Therapeutics (2021)
-
Nanoantibiotics containing membrane-active human cathelicidin LL-37 or synthetic ceragenins attached to the surface of magnetic nanoparticles as novel and innovative therapeutic tools: current status and potential future applications
Journal of Nanobiotechnology (2020)
-
In vitro and intracellular activities of frog skin temporins against Legionella pneumophila and its eukaryotic hosts
Scientific Reports (2020)