Abstract
Background
The objective of this study was to evaluate the comparative efficacy of current interventions for the prevention of retinopathy of prematurity (ROP) in premature infants.
Methods
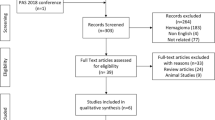
A network meta-analysis (NMA) was performed. We searched PubMed, Web of Science, Scopus, Embase, and the Cochrane Library for relevant studies from their inception to May 5, 2022. Publications were eligible for our study if they were randomized controlled trials (RCTs) involving preterm infants at <37 weeks of gestational age and reported the incidence of any-stage ROP treated with the interventions of interest. The overall effect was pooled using the random effects model.
Results
We identified 106 RCTs (involving 23894 participants). This NMA showed that vitamin A supplementation markedly reduced the incidence of ROP, in comparison with placebo (odds ratio [OR] = 0.59, 95% credible interval [95% CrI] 0.33, 0.85), fish oil-based lipid emulsion (OR = 0.57, 95% CrI 0.24, 0.90), early erythropoietin (OR = 0.51, 95% CrI 0.34, 0.98), probiotics (OR = 0.48, 95% CrI 0.32, 0.97), and human milk (OR = 0.50, 95% CrI 0.21, 0.78). Vitamin A supplementation has the highest probability of being the best option for reducing the ROP risk compared with the other 20 interventions based on its surface under the cumulative ranking curve (SUCRA) value (SUCRA = 92.50%, 95% CrI 0.71, 1.00).
Conclusions
Our findings suggest that among 21 interventions, vitamin A supplementation might be the best method of preventing ROP. This NMA offers an important resource for further efforts to develop preventive strategies for ROP.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 18 print issues and online access
$259.00 per year
only $14.39 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Fang JL, Sorita A, Carey WA, Colby CE, Murad MH, Alahdab F. Interventions to prevent retinopathy of prematurity: a meta-analysis. Pediatrics. 2016;137:e20153387.
Cavallaro G, Filippi L, Bagnoli P, La Marca G, Cristofori G, Raffaeli G, et al. The pathophysiology of retinopathy of prematurity: an update of previous and recent knowledge. Acta Ophthalmol. 2014;92:2–20.
Hack M, Wright LL, Shankaran S, Tyson JE, Horbar JD, Bauer CR, et al. Very-low-birth-weight outcomes of the National Institute of Child Health and Human Development Neonatal Network, November 1989 to October 1990. Am J Obstet Gynecol. 1995;172:457–64.
Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–56.
Lemons JA, Bauer CR, Oh W, Korones SB, Papile LA, Stoll BJ, et al. Very low birth weight outcomes of the National Institute of Child health and human development neonatal research network, January 1995 through December 1996. NICHD Neonatal Research Network. Pediatrics. 2001;107:E1.
Li Z, Zhang Y, Liao Y, Zeng R, Zeng P, Lan Y. Comparison of efficacy between anti-vascular endothelial growth factor (VEGF) and laser treatment in Type-1 and threshold retinopathy of prematurity (ROP). BMC Ophthalmol. 2018;18:19.
Sankar MJ, Sankar J, Chandra P. Anti-vascular endothelial growth factor (VEGF) drugs for treatment of retinopathy of prematurity. Cochrane Database Syst Rev. 2018;1:CD009734.
VanderVeen DK, Melia M, Yang MB, Hutchinson AK, Wilson LB, Lambert SR. Anti-vascular endothelial growth factor therapy for primary treatment of type 1 retinopathy of prematurity: a report by the American Academy of Ophthalmology. Ophthalmology. 2017;124:619–33.
Wheatley CM, Dickinson JL, Mackey DA, Craig JE, Sale MM. Retinopathy of prematurity: recent advances in our understanding. Br J Ophthalmol. 2002;86:696–700.
Chen M, Citil A, McCabe F, Leicht KM, Fiascone J, Dammann CE, et al. Infection, oxygen, and immaturity: interacting risk factors for retinopathy of prematurity. Neonatology. 2011;99:125–32.
Fierson WM, Ophthalmology AAOPSo, American Academy Of O, American Association For Pediatric O, Strabismus, American Association Of Certified O. Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2018;142:e20183061.
Hartnett ME, Lane RH. Effects of oxygen on the development and severity of retinopathy of prematurity. J AAPOS. 2013;17:229–34.
Hartnett ME. Pathophysiology and mechanisms of severe retinopathy of prematurity. Ophthalmology. 2015;122:200–10.
Hauspurg AK, Allred EN, Vanderveen DK, Chen M, Bednarek FJ, Cole C, et al. Blood gases and retinopathy of prematurity: the ELGAN study. Neonatology. 2011;99:104–11.
Lee J, Dammann O. Perinatal infection, inflammation, and retinopathy of prematurity. Semin Fetal Neonatal Med. 2012;17:26–9.
Hellstrom A, Hard AL, Engstrom E, Niklasson A, Andersson E, Smith L, et al. Early weight gain predicts retinopathy in preterm infants: new, simple, efficient approach to screening. Pediatrics. 2009;123:e638–45.
ELFIN trial investigators group. Enteral lactoferrin supplementation for very preterm infants: a randomised placebo-controlled trial. Lancet. 2019;393:423–33.
Fauchère JC, Koller BM, Tschopp A, Dame C, Ruegger C, Bucher HU. Safety of early high-dose recombinant erythropoietin for neuroprotection in very preterm infants. J Pediatr. 2015;167:52–7.e51-3.
Sun H, Cheng R, Wang Z. Early vitamin a supplementation improves the outcome of retinopathy of prematurity in extremely preterm infants. Retina. 2020;40:1176–84.
Cota F, Costa S, Giannantonio C, Purcaro V, Catenazzi P, Vento G. Lutein supplementation and retinopathy of prematurity: a meta-analysis. J Matern Fetal Neonatal Med. 2022;35:175–80.
Du Y, He Y, Wang YL, Zhou JG, Chen C. The efficacy and safety of inositol supplementation in preterm infants to prevent retinopathy of prematurity: a systematic review and meta-analysis. BMC Ophthalmol. 2019;19:135.
Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391:1357–66.
da Costa BR, Pereira TV, Saadat P, Rudnicki M, Iskander SM, Bodmer NS, et al. Effectiveness and safety of non-steroidal anti-inflammatory drugs and opioid treatment for knee and hip osteoarthritis: network meta-analysis. BMJ. 2021;375:n2321.
Anothaisintawee T, Attia J, Nickel JC, Thammakraisorn S, Numthavaj P, McEvoy M, et al. Management of chronic prostatitis/chronic pelvic pain syndrome: a systematic review and network meta-analysis. JAMA. 2011;305:78–86.
Huang J, Wen D, Wang Q, McAlinden C, Flitcroft I, Chen H, et al. Efficacy comparison of 16 interventions for myopia control in children: a network meta-analysis. Ophthalmology. 2016;123:697–708.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–41.
Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–84.
Hellström A, Smith LE, Dammann O. Retinopathy of prematurity. Lancet. 2013;382:1445–57.
Aranda JV, Qu J, Valencia GB, Beharry KD. Pharmacologic interventions for the prevention and treatment of retinopathy of prematurity. Semin Perinatol. 2019;43:360–6.
Filippi L, Dal Monte M. A safety review of drugs used for the treatment of retinopathy of prematurity. Expert Opin Drug Saf. 2020;19:1409–18.
Carlo WA, Finer NN, Walsh MC, Rich W, Gantz MG, Laptook AR, et al. Target ranges of oxygen saturation in extremely preterm infants. N Engl J Med. 2010;362:1959–69.
Australia B-I, United Kingdom Collaborative G, Tarnow-Mordi W, Stenson B, Kirby A, Juszczak E, et al. Outcomes of two trials of oxygen-saturation targets in preterm infants. N Engl J Med. 2016;374:749–60.
Riley RD, Moons KGM, Snell KIE, Ensor J, Hooft L, Altman DG, et al. A guide to systematic review and meta-analysis of prognostic factor studies. BMJ (Clin Res ed). 2019;364:k4597.
Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142.
Ades AE, Sculpher M, Sutton A, Abrams K, Cooper N, Welton N, et al. Bayesian methods for evidence synthesis in cost-effectiveness analysis. PharmacoEconomics. 2006;24:1–19.
Sutton A, Ades AE, Cooper N, Abrams K. Use of indirect and mixed treatment comparisons for technology assessment. PharmacoEconomics. 2008;26:753–67.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clin Res ed). 2003;327:557–60.
Liang JH, Li J, Wu RK, Li JY, Qian S, Jia RX, et al. Effectiveness comparisons of various psychosocial therapies for children and adolescents with depression: a Bayesian network meta-analysis. Eur Child Adolesc Psychiatry. 2021;30:685–97.
Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 2010;29:932–44.
Higgins JP, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods. 2012;3:98–110.
Liang JH, Lu L, Li JY, Qu XY, Li J, Qian S, et al. Contributions of modifiable risk factors to dementia incidence: a Bayesian network analysis. J Am Med Dir Assoc. 2020;21:1592–9.e1513.
Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ (Clin Res ed). 2005;331:897–900.
Mavridis D, Salanti G. A practical introduction to multivariate meta-analysis. Stat Methods Med Res. 2013;22:133–58.
Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64:163–71.
Chaimani A, Salanti G. Using network meta-analysis to evaluate the existence of small-study effects in a network of interventions. Res Synth Methods. 2012;3:161–76.
Senjam SS, Chandra P. Retinopathy of prematurity: addressing the emerging burden in developing countries. J Fam Med Prim Care. 2020;9:2600–5.
Mactier H, Weaver LT. Vitamin A and preterm infants: what we know, what we don’t know, and what we need to know. Arch Dis Child Fetal Neonatal Ed. 2005;90:F103–8.
MacTier H, McCulloch DL, Hamilton R, Galloway P, Bradnam MS, Young D, et al. Vitamin A supplementation improves retinal function in infants at risk of retinopathy of prematurity. J Pediatr. 2012;160:954–9.e951.
Garofoli F, Barilla D, Angelini M, Mazzucchelli I, De Silvestri A, Guagliano R, et al. Oral vitamin A supplementation for ROP prevention in VLBW preterm infants. Ital J Pediatr. 2020;46:77.
Ozkan H, Duman N, Kumral A, Kasap B, Ozer EA, Lebe B, et al. Inhibition of vascular endothelial growth factor-induced retinal neovascularization by retinoic acid in experimental retinopathy of prematurity. Physiol Res. 2006;55:267–75.
Tsai AS, Chou HD, Ling XC, Al-Khaled T, Valikodath N, Cole E, et al. Assessment and management of retinopathy of prematurity in the era of anti-vascular endothelial growth factor (VEGF). Prog Retin Eye Res. 2021;88:101018.
Agostoni C, Buonocore G, Carnielli VP, De Curtis M, Darmaun D, Decsi T, et al. Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2010;50:85–91.
Landman J, Sive A, Heese HD, Van der Elst C, Sacks R. Comparison of enteral and intramuscular vitamin A supplementation in preterm infants. Early Hum Dev. 1992;30:163–70.
Darlow BA, Graham PJ. Vitamin A supplementation for preventing morbidity and mortality in very low birthweight infants. Cochrane Database Syst Rev. 2000; CD000501.
Zhang S, Zhou R, Li B, Li H, Wang Y, Gu X, et al. Caffeine preferentially protects against oxygen-induced retinopathy. FASEB J. 2017;31:3334–48.
Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, et al. Long-term effects of caffeine therapy for apnea of prematurity. N Engl J Med. 2007;357:1893–902.
Hussein MA, Coats DK, Khan H, Paysse EA, Steinkuller PG, Kong L, et al. Evaluating the association of autonomic drug use to the development and severity of retinopathy of prematurity. J AAPOS. 2014;18:332–7.
Davis PG, Schmidt B, Roberts RS, Doyle LW, Asztalos E, Haslam R, et al. Caffeine for Apnea of Prematurity trial: benefits may vary in subgroups. J Pediatr. 2010;156:382–7.
Petrucci R, Zollo G, Curulli A, Marrosu G. A new insight into the oxidative mechanism of caffeine and related methylxanthines in aprotic medium: may caffeine be really considered as an antioxidant? Biochim Biophys Acta Gen Subj. 2018;1862:1781–9.
Liu R, Gang L, Shen X, Xu H, Wu F, Sheng L. Binding characteristics and superimposed antioxidant properties of caffeine combined with superoxide dismutase. ACS Omega. 2019;4:17417–24.
Davis PG. When to start and stop caffeine and why respiratory status matters. Semin Fetal Neonatal Med. 2020;25:101175.
Bahji A, Pierce M, Wong J, Roberge JN, Ortega I, Patten S. Comparative efficacy and acceptability of psychotherapies for self-harm and suicidal behavior among children and adolescents a systematic review and network meta-analysis. JAMA Netw Open. 2021;4:e216614.
Funding
This study was supported by the National Natural Science Foundation of China (82122059) and Suzhou Science and Technology Development Program (SKY2022172).
Author information
Authors and Affiliations
Contributions
Conception and design of the research: HX, GL, and CWP; Acquisition and interpretation of the data: MZ, PCD, and DLL; Statistical analysis and writing of the manuscript: MZ, PCD, and JHL; Critical revision of the manuscript: HX, GL, and CWP.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, M., Duan, PC., Li, DL. et al. Efficacy comparison of 21 interventions to prevent retinopathy of prematurity: a Bayesian network meta-analysis of randomized controlled trials. Eye 38, 877–884 (2024). https://doi.org/10.1038/s41433-023-02796-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-023-02796-2
This article is cited by
-
Probiotics for Preterm Infants—Update 2024
Current Treatment Options in Pediatrics (2024)