Abstract
Objective
To investigate clinical and spectral-domain optical coherence tomography (SD-OCT) biomarkers correlating with pre-injection visual acuity (VA), post-injection VA, and the likelihood of macular oedema (MO) regression following dexamethasone (DEX) implant injection in non-infectious uveitic (NIU) patients.
Methods
Patient data from Uveitis Services in Milan, Paris, and Berlin were analysed. Eligible participants were NIU patients aged >18 years with MO as the primary indication for DEX treatment. SD-OCT scans and clinical data were collected at the time of DEX injection (pre-injection visit) and after 3 months (post-injection visit). Multivariable regression models, adjusted for pre-injection VA and lens status, were employed to explore associations. MO regression was defined as the absence of intraretinal/subretinal fluid at the post-injection visit.
Results
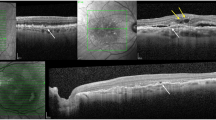
Our analysis comprised data from 173 DEX treatments, encompassing 103 eyes from 80 patients, with 38 eyes (37%) receiving repeated DEX injections. The absence of the ellipsoid zone (EZ) layer and disorganisation of the inner retinal layers (DRIL) were associated with worse pre- (+0.19 LogMAR, 95% CI 0.01–0.38, p = 0.06, and +0.10 LogMAR, 95% CI 0.02–0.21, p = 0.01) and post-injection VA (+0.33 LogMAR, 95% CI 0.08–0.57, p = 0.01, and +0.17 LogMAR, 95% CI 0.01–0.32, p = 0.04). EZ disruption and DRIL increased significantly (p = 0.01 and p = 0.04), and the chance of gaining ≥5 letters declined in eyes undergoing repeated DEX (p = 0.002). The rate of MO regression after each DEX was 67%. Prolonged MO duration (OR = 0.75/each year, p = 0.02) was associated with reduced likelihood of MO regression. Subretinal fluid was associated with higher rate of MO regression (OR = 6.09, p = 0.01).
Conclusion
Integrity of the inner and outer retina is associated with better visual response to DEX. Long-standing or recurrent MO is associated with less chance of both visual and anatomic response. Timely treatment is necessary to maximise the outcomes of MO in NIU patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 18 print issues and online access
$259.00 per year
only $14.39 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Tomkins-Netzer O, Talat L, Bar A, Lula A, Taylor SR, Joshi L, et al. Long-term clinical outcome and causes of vision loss in patients with uveitis. Ophthalmology. 2014;121:2387–92.
Rothova A, Suttorp-van Schulten MS, Frits Treffers W, Kijlstra A. Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol. 1996;80:332–6.
Jabs DA, Rosenbaum JT, Foster CS, Holland GN, Jaffe GJ, Louie JS, et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panel. Am J Ophthalmol. 2000;130:492–513.
Lowder C, Belfort R Jr, Lightman S, Foster CS, Robinson MR, Schiffman RM, et al. Dexamethasone intravitreal implant for noninfectious intermediate or posterior uveitis. Arch Ophthalmol. 2011;129:545–53.
Zarranz-Ventura J, Carreno E, Johnston RL, Mohammed Q, Ross AH, Barker C, et al. Multicenter study of intravitreal dexamethasone implant in noninfectious uveitis: indications, outcomes, and reinjection frequency. Am J Ophthalmol. 2014;158:1136–45.e5.
Khurana RN, Porco TC. Efficacy and safety of dexamethasone intravitreal implant for persistent uveitic cystoid macular edema. Retina. 2015;35:1640–6.
Tomkins-Netzer O, Taylor SR, Bar A, Lula A, Yaganti S, Talat L, et al. Treatment with repeat dexamethasone implants results in long-term disease control in eyes with noninfectious uveitis. Ophthalmology. 2014;121:1649–54.
Tsang AC, Virgili G, Abtahi M, Gottlieb CC. Intravitreal dexamethasone implant for the treatment of macular edema in chronic non-infectious uveitis. Ocul Immunol Inflamm. 2017;25:685–92.
Kang EY, Garg SJ, Chen HF, Wu WC, Chen LY, Chou HD, et al. Intravitreal dexamethasone implants for refractory macular edema in eyes with noninfectious uveitis. J Clin Med. 2021;10:3762.
Pohlmann D, Vom Brocke GA, Winterhalter S, Steurer T, Thees S, Pleyer U. Dexamethasone inserts in noninfectious uveitis: a single-center experience. Ophthalmology. 2018;125:1088–99.
Marchese A, Cicinelli MV, Amato A, Bandello F, Gupta V, Miserocchi E, et al. The next steps in ocular imaging in uveitis. Ocul Immunol Inflamm. 2023;31:1–8.
Ciulla TA, Kapik B, Grewal DS, Ip MS. Visual acuity in retinal vein occlusion, diabetic, and uveitic macular edema: central subfield thickness and ellipsoid zone analysis. Ophthalmol Retin. 2021;5:633–47.
Zur D, Iglicki M, Busch C, Invernizzi A, Mariussi M, Loewenstein A, et al. OCT biomarkers as functional outcome predictors in diabetic macular edema treated with dexamethasone implant. Ophthalmology. 2018;125:267–75.
Cicinelli MV, Chatziralli I, Touhami S, Smaoui A, Tombolini B, Nassisi M, et al. Epiretinal membrane peeling in eyes with retinal vein occlusion: visual and morphologic outcomes. Ophthalmol Ther. 2022;11:661–75.
Rübsam A, Wernecke L, Rau S, Pohlmann D, Müller B, Zeitz O, et al. Behavior of SD-OCT detectable hyperreflective foci in diabetic macular edema patients after therapy with anti-VEGF agents and dexamethasone implants. J Diabetes Res. 2021;2021:8820216.
Zhang Z, Yuan KH. Practical Statistical power analysis using webpower and R. Granger: ISDSA Press; 2018.
Grewal DS, O’Sullivan ML, Kron M, Jaffe GJ. Association of disorganization of retinal inner layers with visual acuity in eyes with uveitic cystoid macular edema. Am J Ophthalmol. 2017;177:116–25.
Tibshirani R. Regression shrinkage and selection via the lasso. J R Stat Soc Ser B Methodol. 1996;58:267–88.
Khurana RN, Bansal AS, Chang LK, Palmer JD, Wu C, Wieland MR. Prospective evaluation of a sustained-release dexamethasone intravitreal implant for cystoid macular edema in quiescent uveitis. Retina. 2017;37:1692–9.
Cao JH, Mulvahill M, Zhang L, Joondeph BC, Dacey MS. Dexamethasone intravitreal implant in the treatment of persistent uveitic macular edema in the absence of active inflammation. Ophthalmology. 2014;121:1871–6.
Matas J, Llorenç V, Fonollosa A, Esquinas C, Diaz-Valle D, Berasategui B, et al. Predictors for functional and anatomic outcomes in macular edema secondary to non-infectious uveitis. PLoS ONE. 2019;14:e0210799.
Habot-Wilner Z, Sorkin N, Goldenberg D, Loewenstein A, Goldstein M. Long-term outcome of an intravitreal dexamethasone implant for the treatment of noninfectious uveitic macular edema. Ophthalmologica. 2014;232:77–82.
Miserocchi E, Modorati G, Pastore MR, Bandello F. Dexamethasone intravitreal implant: an effective adjunctive treatment for recalcitrant noninfectious uveitis. Ophthalmologica. 2012;228:229–33.
Panozzo G, Cicinelli MV, Augustin AJ, Battaglia Parodi M, Cunha-Vaz J, Guarnaccia G, et al. An optical coherence tomography-based grading of diabetic maculopathy proposed by an international expert panel: the European School for Advanced Studies in Ophthalmology classification. Eur J Ophthalmol. 2020;30:8–18.
Sun JK, Lin MM, Lammer J, Prager S, Sarangi R, Silva PS, et al. Disorganization of the retinal inner layers as a predictor of visual acuity in eyes with center-involved diabetic macular edema. JAMA Ophthalmol. 2014;132:1309–16.
Cavalleri M, Cicinelli MV, Parravano M, Varano M, De Geronimo D, Sacconi R, et al. Prognostic role of optical coherence tomography after switch to dexamethasone in diabetic macular edema. Acta Diabetol. 2020;57:163–71.
Berry D, Thomas AS, Fekrat S, Grewal DS. Association of disorganization of retinal inner layers with ischemic index and visual acuity in central retinal vein occlusion. Ophthalmol Retin. 2018;2:1125–32.
Zur D, Iglicki M, Feldinger L, Schwartz S, Goldstein M, Loewenstein A, et al. Disorganization of retinal inner layers as a biomarker for idiopathic epiretinal membrane after macular surgery—the DREAM Study. Am J Ophthalmol. 2018;196:129–35.
Ciulla TA, Kapik B, Barakat MR, Khurana RN, Nguyen QD, Grewal DS, et al. Optical coherence tomography anatomic and temporal biomarkers in uveitic macular edema. Am J Ophthalmol. 2022;237:310–24.
Bodaghi B, Brézin AP, Weber M, Delcourt C, Kodjikian L, Provost A, et al. Real-life efficacy, safety, and use of dexamethasone intravitreal implant in posterior segment inflammation due to non-infectious uveitis (LOUVRE 2 Study). Ophthalmol Ther. 2022;11:1775–92.
Jonas JB, Spandau UH, Kamppeter BA, Vossmerbaeumer U, Harder B, Sauder G. Repeated intravitreal high-dosage injections of triamcinolone acetonide for diffuse diabetic macular edema. Ophthalmology. 2006;113:800–4.
Nicholson BP, Zhou M, Rostamizadeh M, Mehta P, Agrón E, Wong W, et al. Epidemiology of epiretinal membrane in a large cohort of patients with uveitis. Ophthalmology. 2014;121:2393–8.
Rao P, Todorich B, Yonekawa Y, Wang J, Sobrin L, Faia LJ. Surgical outcomes of epiretinal membranes in patients with a history of well-controlled preoperative uveitis. Ophthalmol Retin. 2018;2:192–6.
Munk MR, Bolz M, Huf W, Sulzbacher F, Roberts P, Simader C, et al. Morphologic and functional evaluations during development, resolution, and relapse of uveitis-associated cystoid macular edema. Retina. 2013;33:1673–83.
Berasategui B, Fonollosa A, Artaraz J, Ruiz-Arruza I, Ríos J, Matas J, et al. Behavior of hyperreflective foci in non-infectious uveitic macular edema, a 12-month follow-up prospective study. BMC Ophthalmol. 2018;18:179.
Lehpamer B, Moshier E, Goldberg N, Ackert J, Godbold J, Jabs DA. Subretinal fluid in uveitic macular edema: effect on vision and response to therapy. Am J Ophthalmol. 2013;155:143–9.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Financial disclosures: CG, PS, EM: no financial disclosures. MVC, ST, DP, AG, AR previously received travel grants from Allergan as International Retinal Panel members. FB consultant for: Allergan Inc (Irvine, California, USA), Bayer Schering-Pharma (Berlin, Germany), Hoffmann-La-Roche (Basel, Switzerland), Novartis (Basel, Switzerland), Sanofi-Aventis (Paris, France), Thrombogenics (Heverlee, Belgium), Zeiss (Dublin, USA), Boehringer-Ingelheim, Fidia Sooft, NTC Pharma, Sifi. AL consultant for: Allergan, Bayer health care, Beyeonics, ForSight Labs, Notal Vision, Novartis, Roche, WebMD, Syneos, Xbrane, Nanoretina, Ocuterra, Ripple Therapeutics, Annexon, MJHEvents, Iveric Bio, Biogen, Johnson & Johnson, Ophtimedrx, Ocuphire Pharma, Iqvia.
Author information
Authors and Affiliations
Consortia
Contributions
All the authors contributed to the conception or design of the work, the acquisition, analysis, and interpretation of data, drafting the work, and revising it critically for intellectual content. Each coauthor has seen and agrees with how his or her name is listed.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cicinelli, M.V., Gerosolima, C., Scandale, P. et al. Clinical and imaging biomarkers of response to intravitreal dexamethasone implant in eyes with non-infectious uveitic macular oedema. Eye 38, 910–916 (2024). https://doi.org/10.1038/s41433-023-02802-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-023-02802-7