Abstract
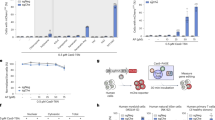
B-cell lines and primary PBMCs are notoriously hard to transfect, thus making genome editing, ectopic gene expression, or gene silencing experiments particularly tedious. Here we propose a novel efficient and reproducible protocol for electrotransfection of lymphoblastoid, B-cell lymphoma, leukemia cell lines, and B cells from PBMCs. The proposed protocol requires neither costly equipment nor expensive reagents; it can be used with small or large plasmids. Transfection and viability rates of about 79% and 58%, respectively, have been routinely achieved by optimizing the salt concentration in the electrotransfection medium and the amount of plasmid used. A validation of the protocol was obtained via the generation of a TP53−/− RPMI8866 lymphoblastoid cell line which should prove useful in future hematological and blood cancer studies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Zhao N, Qi J, Zeng Z, Parekh P, Chang CC, Tung CH, et al. Transfecting the hard-to-transfect lymphoma/leukemia cells using a simple cationic polymer nanocomplex. J Control Rel. 2012;159:104–10.
Meacham JM, Durvasula K, Degertekin FL, Fedorov AG. Physical methods for intracellular delivery. J Lab Autom. 2014;19:1–18.
Kaestner L, Scholz A, Lipp P. Conceptual and technical aspects of transfection and gene delivery. Bioorg Med Chem Let. 2015;25:1171–6.
Mosier DE. Introduction for “Safety considerations for retroviral vectors: a short review.”. Appl Biosaf. 2016;9:68–75.
Glover DJ, Lipps HJ, Jans DA. Towards safe, non-viral therapeutic gene expression in humans. Nat Rev Genet. 2005;6:299–310.
Kim TK, Eberwine JH. Mammalian cell transfection: the present and the future. Anal Bioanal Chem. 2010;397:3173–8.
Rols M-P. Electropermeabilization, a physical method for the delivery of therapeutic molecules into cells. Biochim et Biophys Acta—Biomemb. 2006;1758:423–8.
Jordan ET, Collins M, Terefe J, Ugozzoli L, Rubio T. Optimizing electroporation conditions in primary and other difficult-to-transfect cells. J Biomol Tech. 2008;19:328–34.
Chicaybam L, Barcelos C, Peixoto B, Carneiro M, Limia CG, Redondo P, et al. An efficient electroporation protocol for the genetic modification of mammalian cells. Front Bioeng Biotech. 2016;4:99.
Machy P, Lewis F, McMillan L, Jonak ZL. Gene transfer from targeted liposomes to specific lymphoid cells by electroporation. Proc Nat Acad Sci. 2006;85:8027–31.
Maurisse R, De Semir D, Emamekhoo H, Bedayat B, Abdolmohammadi A, Parsi H, et al. Comparative transfection of DNA into primary and transformed mammalian cells from different lineages. BMC Biotechnol. 2010;10:9.
Gahn TA, Sugden B. Marked, transient inhibition of expression of the Epstein-Barr virus latent membrane protein gene in Burkitt’s lymphoma cell lines by electroporation. J Virol. 1993;67:6379–86.
Goldstein S, Fordis CM, Howard BH. Enhanced transfection efficiency and improved cell survival after electroporation of G2/M-synchronized cells and treatment with sodium butyrate. Nucleic Acids Res. 1989;17:3959–71.
Liew A, André FM, Lesueur LL, De Ménorval M-A, O’Brien T, Mir LM. Robust, efficient, and practical electrogene transfer method for human mesenchymal stem cells using square electric pulses. Hum Gene Ther Methods. 2013;24:289–97.
Silve A, Leray I, Poignard C, Mir LM. Impact of external medium conductivity on cell membrane electropermeabilization by microsecond and nanosecond electric pulses. Sci Rep. 2016;6:19957.
Kreiss P, Cameron B, Rangara R, Mailhe P, Aguerre-Charriol O, Airiau M, et al. Plasmid DNA size does not affect the physicochemical properties of lipoplexes but modulates gene transfer efficiency. Nucleic Acids Res. 1999;27:3792–8.
Lesueur LL, Mir LM, André FM. Overcoming the specific toxicity of large plasmids electrotransfer in primary cells in vitro. Mol Therapy - Nucleic Acids. 2016;5:e291.
Germini D, Saada YB, Tsfasman T, Osina K, Robin CC, Lomov N, et al. A one-step PCR-based assay to evaluate the efficiency and precision of genomic DNA-editing tools. Mol Ther Meth Clin De. 2017;5:43–50.
Georgakilas AG, Martin OA, Bonner WM. p21: a two-faced genome guardian. Trends Mol Med. 2017;23:310–9.
Acknowledgements
This research was supported by grants from the GEFLUC, LNCC, the Presidium of RAS and the State program of fundamental scientific research of IDB RAS. We gratefully acknowledge the technical support of the imaging and cytometry platform of the Gustave Roussy Institute.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Canoy, R.J., André, F., Shmakova, A. et al. Easy and robust electrotransfection protocol for efficient ectopic gene expression and genome editing in human B cells. Gene Ther 30, 167–171 (2023). https://doi.org/10.1038/s41434-020-00194-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41434-020-00194-x
This article is cited by
-
Transcriptional effects of electroporation on Echinococcus multilocularis primary cell culture
Parasitology Research (2022)