Abstract
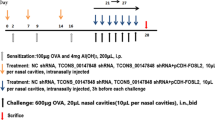
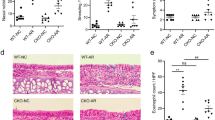
Recent studies have revealed that YKL-40 is involved in the pathogenesis of asthma. However, its specific mechanism remains unclear. The present study aims to investigate the effect of adenovirus vector-mediated YKL-40 short hairpin RNA (shRNA) on regulation of airway inflammation in a murine asthmatic model. Mice were assessed for airway hyperresponsiveness (AHR), total leukocytes and the percentage of eosinophil cells in bronchoalveolar lavage fluid (BALF). YKL-40 mRNA and protein expression levels were detected using quantitative real-time PCR and western blot assays. Enzyme-linked immunosorbent assay (ELISA) was used to detect YKL-40 and eosinophil-related chemokine expression levels in BALF and serum. Lung histology analyses were performed to evaluate the degree of inflammatory cell infiltration around the airway and airway mucus secretion.YKL-40 shRNA significantly inhibited the YKL-40 gene expression in asthmatic mice. In addition, YKL-40 shRNA alleviated eosinophilic airway inflammation, AHR, airway mucus secretion and decreased the levels of YKL-40 in BALF and serum in a murine asthmatic model. The levels and mRNA expression of IL-5, IL-13 in asthmatic mice lung tissues, eotaxin, and GM-CSF in BALF and serum significantly decreased. Bone marrow signaling molecules including IL-5, eotaxin, and GM-CSF were correlated with decreased levels of YKL-40. The study reveals that YKL-40 could be involved in asthma inflammation by altering bone marrow signaling molecules. YKL-40 gene RNA interference could provide new therapeutic strategies for asthma.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Komi DE, Kazemi T, Bussink AP. New insights into the relationship between chitinase-3-like-1 and asthma. Curr Allergy Asthma Rep. 2016;16:57.
Davoine F, Lacy P. Eosinophil cytokines, chemokines, and growth factors: emerging roles in immunity. Front Immunol. 2014;5:570.
Kulkarni NS, Hollins F, Sutcliffe A, Saunders R, Shah S, Siddiqui S, et al. Eosinophil protein in airway macrophages: a novel biomarker of eosinophilic inflammation in patients with asthma. J Allergy Clin Immunol. 2010;126:61–9.e3.
Lambrecht BN, Hammad H. The role of dendritic and epithelial cells as master regulators of allergic airway inflammation. Lancet. 2010;376:835–43.
Lopez AF, Williamson DJ, Gamble JR, Begley CG, Harlan JM, Klebanoff SJ, et al. Recombinant human granulocyte-macrophage colony-stimulating factor stimulates in vitro mature human neutrophil and eosinophil function, surface receptor expression, and survival. J Clin Invest. 1986;78:1220–8.
Denburg JA, Sehmi R, Saito H, Pil-Seob J, Inman MD, O’Byrne PM. Systemic aspects of allergic disease: bone marrow responses. J Allergy Clin Immunol. 2000;106:S242–6.
Luhadia SK. Steroid resistant asthma. J Assoc Physicians India. 2014;62:38–40.
Hakala BE, White C, Recklies AD. Human cartilage gp-39, a major secretory product of articular chondrocytes and synovial cells, is a mammalian member of a chitinase protein family. J Biol Chem. 1993;268:25803–10.
Rehli M, Krause SW, Andreesen R. Molecular characterization of the gene for human cartilage gp-39 (CHI3L1), a member of the chitinase protein family and marker for late stages of macrophage differentiation. Genomics. 1997;43:221–5.
Shackelton LM, Mann DM, Millis AJ. Identification of a 38-kDa heparin-binding glycoprotein (gp38k) in differentiating vascular smooth muscle cells as a member of a group of proteins associated with tissue remodeling. J Biol Chem. 1995;270:13076–83.
Bigg HF, Wait R, Rowan AD, Cawston TE. The mammalian chitinase-like lectin, YKL-40, binds specifically to type I collagen and modulates the rate of type I collagen fibril formation. J Biol Chem. 2006;281:21082–95.
Recklies AD, White C, Ling H. The chitinase 3-like protein human cartilage glycoprotein 39 (HC-gp39) stimulates proliferation of human connective-tissue cells and activates both extracellular signal-regulated kinase- and protein kinase B-mediated signalling pathways. Biochem J. 2002;365:119–26.
Rusak A, Jablonska K, Dziegiel P. The role of YKL-40 in a cancerous process. Postepy Hig Med Dosw (Online). 2016;70:1286–99.
Hansen JW, Thomsen SF, Porsbjerg C, Rasmussen LM, Harmsen L, Johansen JS, et al. YKL-40 and genetic status of CHI3L1 in a large group of asthmatics. Eur Clin Respir J. 2015;2:25117.
Usemann J, Frey U, Mack I, Schmidt A, Gorlanova O, Roosli M, et al. CHI3L1 polymorphisms, cord blood YKL-40 levels and later asthma development. BMC Pulm Med. 2016;16:81.
Hartl D, Lee CG, Da Silva CA, Chupp GL, Elias JA. Novel biomarkers in asthma: chemokines and chitinase-like proteins. Curr Opin Allergy Clin Immunol. 2009;9:60–6.
Lai T, Chen M, Deng Z, Wu LY, Li D., et al. YKL-40 is correlated with FEV1 and the asthma control test (ACT) in asthmatic patients: influence of treatment. BMC Pulm Med. 2015;15:1.
Lee CG, Da Silva CA, Dela Cruz CS, Ahangari F, Ma B, Kang MJ, et al. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu Rev Physiol. 2011;73:479–501.
Ober C, Tan Z, Sun Y, Possick JD, Pan L, Nicolae R, et al. Effect of variation in CHI3L1 on serum YKL-40 level, risk of asthma, and lung function. N Engl J Med. 2008;358:1682–91.
Tang H, Shi Z, Xiu Q, Li B, Sun Y. YKL-40-mediated interleukin 8 production may be closely associated with remodeling of bronchial smooth muscle cells. Am J Respir Crit Care Med. 2012;186:386. author reply -7
Chupp GL, Lee CG, Jarjour N, Shim YM, Holm CT, He S, et al. A chitinase-like protein in the lung and circulation of patients with severe asthma. N Engl J Med. 2007;357:2016–27.
Lee CG, Elias JA. Role of breast regression protein-39/YKL-40 in asthma and allergic responses. Allergy Asthma Immunol Res. 2010;2:20–7.
Ben SQ, Qiu YL, Zhou J, Zhou XY, Zhang S, Wu Y, et al. Ovalbumin enhances YKL-40, IL-5, GM-CSF, and eotaxin expression simultaneously in primarily cultured mouse tracheal epithelial cells. In Vitro Cell Dev Biol Anim. 2014;50:243–50.
Khanna K, Chaudhuri R, Aich J, Pattnaik B, Panda L, Prakash YS, et al. Secretory inositol polyphosphate 4-phosphatase protects against airway inflammation and remodeling. Am J Respir Cell Mol Biol. 2019;60:399–412.
Bao W, Zhang Y, Zhang M, Bao A, Fei X, Zhang X, et al. Effects of ozone repeated short exposures on the airway/lung inflammation, airway hyperresponsiveness and mucus production in a mouse model of ovalbumin-induced asthma. Biomed Pharmacother. 2018;101:293–303.
Fei X, Bao W, Zhang P, Zhang X, Zhang G, Zhang Y, et al. Inhalation of progesterone inhibits chronic airway inflammation of mice exposed to ozone. Mol Immunol. 2017;85:174–84.
McMillan SJ, Xanthou G, Lloyd CM. Therapeutic administration of Budesonide ameliorates allergen-induced airway remodelling. Clin Exp Allergy. 2005;35:388–96.
Rathcke CN, Raymond I, Kistorp C, Hildebrandt P, Faber J, Vestergaard H. Low grade inflammation as measured by levels of YKL-40: association with an increased overall and cardiovascular mortality rate in an elderly population. Int J Cardiol. 2010;143:35–42.
Kastrup J, Johansen JS, Winkel P, Hansen JF, Hildebrandt P, Jensen GB, et al. High serum YKL-40 concentration is associated with cardiovascular and all-cause mortality in patients with stable coronary artery disease. Eur Heart J. 2009;30:1066–72.
Rathcke CN, Persson F, Tarnow L, Rossing P, Vestergaard H. YKL-40, a marker of inflammation and endothelial dysfunction, is elevated in patients with type 1 diabetes and increases with levels of albuminuria. Diabetes Care. 2009;32:323–8.
Bonneh-Barkay D, Wang G, Laframboise WA, Wiley CA, Bissel SJ. Exacerbation of experimental autoimmune encephalomyelitis in the absence of breast regression protein 39/chitinase 3-like 1. J Neuropathol Exp Neurol. 2012;71:948–58.
Kazakova M, Batalov A, Deneva T, Mateva N, Kolarov Z, Sarafian V. Relationship between sonographic parameters and YKL-40 levels in rheumatoid arthritis. Rheumatol Int. 2013;33:341–6.
Sekine T, Masuko-Hongo K, Matsui T, Asahara H, Takigawa M, Nishioka K, et al. Recognition of YKL-39, a human cartilage related protein, as a target antigen in patients with rheumatoid arthritis. Ann Rheum Dis. 2001;60:49–54.
Suzuki H, Boki H, Kamijo H, Nakajima R, Oka T, Shishido-Takahashi N, et al. YKL-40 promotes proliferation of cutaneous T-cell lymphoma tumor cells through extracellular signal-regulated kinase pathways. J Invest Dermatol. 2020;140:860–8.
Wang H, Long XB, Cao PP, Wang N, Liu Y, Cui YH, et al. Clara cell 10-kD protein suppresses chitinase 3-like 1 expression associated with eosinophilic chronic rhinosinusitis. Am J Respir Crit Care Med. 2010;181:908–16.
Tang H, Sun Y, Shi Z, Huang H, Fang Z, Chen J, et al. YKL-40 induces IL-8 expression from bronchial epithelium via MAPK (JNK and ERK) and NF-kappaB pathways, causing bronchial smooth muscle proliferation and migration. J Immunol. 2013;190:438–46.
Ortega H, Prazma C, Suruki RY, Li H, Anderson WH. Association of CHI3L1 in African-Americans with prior history of asthma exacerbations and stress. J Asthma. 2013;50:7–13.
Naglot S, Aggarwal P, Dey S, Dalal K. Estimation of serum YKL-40 by real-time surface plasmon resonance technology in North-Indian asthma patients. J Clin Lab Anal. 2017;31:e22028. https://doi.org/10.1002/jcla.22028.
Lee CG, Dela Cruz CS, Herzog E, Rosenberg SM, Ahangari F, Elias JA. YKL-40, a chitinase-like protein at the intersection of inflammation and remodeling. Am J Respir Crit Care Med. 2012;185:692–4.
Lee CG, Hartl D, Lee GR, Koller B, Matsuura H, Da Silva CA, et al. Role of breast regression protein 39 (BRP-39)/chitinase 3-like-1 in Th2 and IL-13-induced tissue responses and apoptosis. J Exp Med. 2009;206:1149–66.
Xu Q, Chai SJ, Qian YY, Zhang M, Wang K. Breast regression protein-39 (BRP-39) promotes dendritic cell maturation in vitro and enhances Th2 inflammation in murine model of asthma. Acta Pharmacol Sin. 2012;33:1525–32.
Iwasaki H, Mizuno S, Mayfield R, Shigematsu H, Arinobu Y, Seed B, et al. Identification of eosinophil lineage-committed progenitors in the murine bone marrow. J Exp Med. 2005;201:1891–7.
Ben S, Li X, Xu F, Xu W, Li W, Wu Z, et al. Treatment with anti-CC chemokine receptor 3 monoclonal antibody or dexamethasone inhibits the migration and differentiation of bone marrow CD34 progenitor cells in an allergic mouse model. Allergy. 2008;63:1164–76.
Salter BM, Sehmi R. Hematopoietic processes in eosinophilic asthma. Chest. 2017;152:410–6.
Nobs SP, Kayhan M, Kopf M. GM-CSF intrinsically controls eosinophil accumulation in the setting of allergic airway inflammation. J Allergy Clin Immunol. 2019;143:1513–24.e2.
Allakhverdi Z, Comeau MR, Smith DE, Toy D, Endam LM, Desrosiers M, et al. CD34+ hemopoietic progenitor cells are potent effectors of allergic inflammation. J Allergy Clin Immunol. 2009;123:472–8.
Allakhverdi Z, Delespesse G. Hematopoietic progenitor cells are innate Th2 cytokine-producing cells. Allergy. 2012;67:4–9.
Foster PS, Maltby S, Rosenberg HF, Tay HL, Hogan SP, Collison AM, et al. Modeling T(H) 2 responses and airway inflammation to understand fundamental mechanisms regulating the pathogenesis of asthma. Immunol Rev. 2017;278:20–40.
Kumar RK, Thomas PS, Seetoo DQ, Herbert C, McKenzie AN, Foster PS, et al. Eotaxin expression by epithelial cells and plasma cells in chronic asthma. Lab Invest. 2002;82:495–504.
Han M, Hu R, Ma J, Zhang B, Chen C, Li H, et al. Fas signaling in dendritic cells mediates Th2 polarization in HDM-induced allergic pulmonary inflammation. Front Immunol. 2018;9:3045.
Gomez JL, Yan X, Holm CT, Grant N, Liu Q, Cohn L, et al. Characterisation of asthma subgroups associated with circulating YKL-40 levels. Eur Respir J. 2017;50:1700800. https://doi.org/10.1183/13993003.00800-2017.
Li TM, Liu SC, Huang YH, Huang CC, Hsu CJ, Tsai CH, et al. YKL-40-induced inhibition of miR-590-3p promotes interleukin-18 expression and angiogenesis of endothelial progenitor cells. Int J Mol Sci. 2017;18:920. https://doi.org/10.3390/ijms18050920.
Kang MJ, Yoon CM, Nam M, Kim DH, Choi JM, Lee CG, et al. Role of chitinase 3-like-1 in interleukin-18-induced pulmonary type 1, type 2, and type 17 inflammation; alveolar destruction; and airway fibrosis in the murine lung. Am J Respir Cell Mol Biol. 2015;53:863–71.
Kandikattu HK, Upparahalli Venkateshaiah S, Mishra A. Synergy of interleukin (IL)-5 and IL-18 in eosinophil mediated pathogenesis of allergic diseases. Cytokine Growth Factor Rev. 2019;47:83–98.
Sawada M, Kawayama T, Imaoka H, Sakazaki Y, Oda H, Takenaka S, et al. IL-18 induces airway hyperresponsiveness and pulmonary inflammation via CD4+ T cell and IL-13. PLoS One. 2013;8:e54623.
Xu MH, Yuan FL, Wang SJ, Xu HY, Li CW, Tong X. Association of interleukin-18 and asthma. Inflammation. 2017;40:324–7.
Leggiero E, Labruna G, Iaffaldano L, Lombardo B, Greco A, Fiorenza D, et al. Helper-dependent adenovirus-mediated gene transfer of a secreted LDL receptor/transferrin chimeric protein reduces aortic atherosclerosis in LDL receptor-deficient mice. Gene Ther. 2019;26:121–30.
Zabner J, Petersen DM, Puga AP, Graham SM, Couture LA, Keyes LD, et al. Safety and efficacy of repetitive adenovirus-mediated transfer of CFTR cDNA to airway epithelia of primates and cotton rats. Nat Genet. 1994;6:75–83.
Zhang WW, Alemany R, Wang J, Koch PE, Ordonez NG, Roth JA. Safety evaluation of Ad5CMV-p53 in vitro and in vivo. Hum Gene Ther. 1995;6:155–64.
Chen L, Zheng J, Xue Q, Zhao Y. YKL-40 promotes the progress of atherosclerosis independent of lipid metabolism in apolipoprotein E(-/-) mice fed a high-fat diet. Heart Vessels. 2019;34:1874–81.
Liu L, Zhang X, Liu Y, Zhang L, Zheng J, Wang J, et al. Chitinase-like protein YKL-40 correlates with inflammatory phenotypes, anti-asthma responsiveness and future exacerbations. Respir Res. 2019;20:95.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No: 81570018 and No: 81970022).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, L., Bao, A., Zheng, Y. et al. Adenovirus vector-mediated YKL-40 shRNA attenuates eosinophil airway inflammation in a murine asthmatic model. Gene Ther 28, 177–185 (2021). https://doi.org/10.1038/s41434-020-00202-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41434-020-00202-0