Abstract
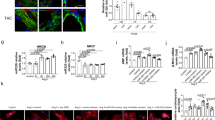
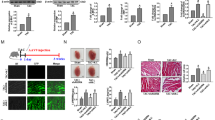
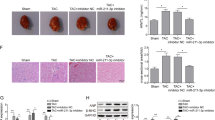
Cardiac hypertrophy is imposed much pressure on heart and threatening our live. Previous study suggested that dysregulation of Celf1 is largely connecting to neonatal cardiac dysfunction. Hence, we aimed to explore the precise function and probable regulatory mechanism upstream of Celf1in cardiac hypertrophy. Here, Ang-II treatment was implemented to stimulate hypertrophic phenotypes inH9C2 and MCM cells. Immunofluorescence assay was conducted to measure the surface area of cardiomyocytes. And qRT-PCR assay was conducted to investigate gene expression. Moreover, western blot assay was conducted to probe the protein levels. Results uncovered that Celf1 expression was increased dependent on elevated Ang-II concentration, and that inhibited Celf1 could relieve the Ang-II-caused cardiac hypertrophy. Significantly, Celf1was found to be targeted by miR-129-5p but then released via the sponging role of circ-Jarid2. Furthermore, circ-Jarid2 was found to promote cardiac hypertrophy, whereas miR-129-5p played suppressing parts in hypertrophic cardiomyocytes. Moreover, we verified circ-Jarid2 contributed to cardiac hypertrophy via miR-129-5p/Celf1 axis both in vitro and in vivo. In conclusion, circ-Jarid2/miR-129-5p/Celf1 axis aggravates cardiac hypertrophy, which provides new ideas for developing treatment strategies for patients with cardiac hypertrophy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
McMurray JJ, Pfeffer MA. Heart failure. Lancet (London, England). 2005;365:1877–89.
Neubauer S. The failing heart–an engine out of fuel. N Engl J Med. 2007;356:1140–51.
Artham SM, Lavie CJ, Milani RV, Patel DA, Verma A, Ventura HO. Clinical impact of left ventricular hypertrophy and implications for regression. Prog Cardiovasc Dis. 2009;52:153–67.
Kavey RE. Left ventricular hypertrophy in hypertensive children and adolescents: predictors and prevalence. Current Hypertens Rep. 2013;15:453–7.
Chung E, Leinwand LA. Pregnancy as a cardiac stress model. Cardiovasc Res. 2014;101:561–70.
Ellison GM, Waring CD, Vicinanza C, Torella D. Physiological cardiac remodelling in response to endurance exercise training: cellular and molecular mechanisms. Heart (Brit Cardiac Soc). 2012;98:5–10.
Huang ZP, Chen J, Seok HY, Zhang Z, Kataoka M, Hu X, et al. MicroRNA-22 regulates cardiac hypertrophy and remodeling in response to stress. Circ Res. 2013;112:1234–43.
Li Z, Song Y, Liu L, Hou N, An X, Zhan D, et al. miR-199a impairs autophagy and induces cardiac hypertrophy through mTOR activation. Cell Death Differ. 2017;24:1205–13.
Chen C, Ponnusamy M, Liu C, Gao J, Wang K, Li P. MicroRNA as a therapeutic target in cardiac remodeling. BioMed Res Int. 2017;2017:1278436.
Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–33.
He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–31.
Blech-Hermoni Y, Sullivan CB, Jenkins MW, Wessely O, Ladd AN. CUG-BP, Elav-like family member 1 (CELF1) is required for normal myofibrillogenesis, morphogenesis, and contractile function in the embryonic heart. Dev Dyn Official Publ Am Assoc Anat. 2016;245:854–73.
Giudice J, Xia Z, Li W, Cooper TA. Neonatal cardiac dysfunction and transcriptome changes caused by the absence of Celf1. Sci Rep. 2016;6:35550.
Peng X, Shen X, Chen X, Liang R, Azares AR, Liu Y. Celf1 regulates cell cycle and is partially responsible for defective myoblast differentiation in myotonic dystrophy RNA toxicity. Biochimica et biophysica acta. 2015;1852:1490–7.
Blech-Hermoni Y, Dasgupta T, Coram RJ, Ladd AN. Identification of targets of CUG-BP, elav-like family member 1 (CELF1) regulation in embryonic heart muscle. PloS one. 2016;11:e0149061.
van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science. 2007;316:575–9.
Callis TE, Pandya K, Seok HY, Tang RH, Tatsuguchi M, Huang ZP, et al. MicroRNA-208a is a regulator of cardiac hypertrophy and conduction in mice. J Clin Investig. 2009;119:2772–86.
Wang J, Song Y, Zhang Y, Xiao H, Sun Q, Hou N, et al. Cardiomyocyte overexpression of miR-27b induces cardiac hypertrophy and dysfunction in mice. Cell Res. 2012;22:516–27.
Zhang H, Zhang X, Zhang J. MiR-129-5p inhibits autophagy and apoptosis of H9c2 cells induced by hydrogen peroxide via the PI3K/AKT/mTOR signaling pathway by targeting ATG14. Biochem Biophys Res Commun. 2018;506:272–7.
Majumdar G, Raghow R. Trichostatin A induces a unique set of microRNAs including miR-129-5p that blocks cyclin-dependent kinase 6 expression and proliferation in H9c2 cardiac myocytes. Mol Cell Biochem. 2016;415:39–49.
Kristensen LS, Andersen MS, Stagsted LVW, Ebbesen KK, Hansen TB, Kjems J. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet. 2019;20:675–91.
Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, et al. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–8.
Cho E, Mysliwiec MR, Carlson CD, Ansari A, Schwartz RJ, Lee Y. Cardiac-specific developmental and epigenetic functions of Jarid2 during embryonic development. J Biol Chem. 2018;293:11659–73.
Xu J, Wang Y, Tan X, Jing H. MicroRNAs in autophagy and their emerging roles in crosstalk with apoptosis. Autophagy. 2012;8:873–82.
Acknowledgements
We appreciate the experimenters.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fang, Y., Tao, Y., Zhou, H. et al. Promoting role of circ-Jarid2/miR-129-5p/Celf1 axis in cardiac hypertrophy. Gene Ther 28, 718–728 (2021). https://doi.org/10.1038/s41434-020-0165-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41434-020-0165-5
This article is cited by
-
Curriculum vitae of CUG binding protein 1 (CELF1) in homeostasis and diseases: a systematic review
Cellular & Molecular Biology Letters (2024)
-
microRNA-206 Suppresses Cholangiocarcinoma Cell Growth and Invasion by Targeting Jumonji AT-Rich Interactive Domain 2
Digestive Diseases and Sciences (2022)