Abstract
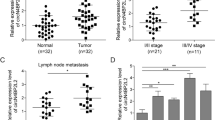
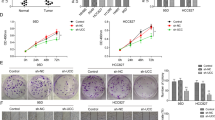
Colorectal cancer (CRC) has been the third leading cause of cancer-associated deaths. LncRNA SNHG16 is reported to be involved in metastasis of CRC cells. However, the mechanism by which SNHG16 regulates CRC progression is poorly understood. The proliferation of CRC cells was examined by MTT. Wound healing and transwell assay were used to measure migration and invasion ability. RT-qPCR and western blot were used to examine gene expression. Immunofluorescence was conducted to evaluate the EMT of CRC cells. Luciferase reporter assay were used to confirm direct interaction between miR-124-3p and SNHG16 or MCP-1. The interaction between miR-124-3p and SNHG16 was detected by RIP and RNA pull down assay. H&E staining was used to test the histomorphological changes of hepatic metastatic nodules. Finally, xenograft tumor experiment was utilized to determine tumor growth in vivo. SNHG16 and miR-124-3p were dysregulated in human colorectal tumors or cells. Knockdown of SNHG16 led to attenuate cell proliferation, migration, invasion, and EMT of CRC cells. And xenograft tumor experiment showed that SNHG16 might influence tumor growth. In contrast, miR-124-3p exerted the antitumor effects. Knockdown of miR-124-3p can reverse the effect of sh-SNHG16 on CRC cells. miR-124-3p could directly bind to SNHG16 or MCP-1. More importantly, MCP-1 acts as a critical effector mediating the role of SNHG16/ miR-124-3p in CRC cells. In summary, our data suggest that SNHG16 plays a contributory role in proliferation, migration, and EMT of CRC cells via miR-124-3p/MCP-1 axis, which offers a rationale for targeting SNHG16 and developing therapeutic drugs to treat CRC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108.
Han D, Wang M, Ma N, Xu Y, Jiang Y, Gao X. Long noncoding RNAs: novel players in colorectal cancer. Cancer Lett. 2015;361:13–21.
Tomida C, Aibara K, Yamagishi N, Yano C, Nagano H, Abe T, et al. The malignant progression effects of regorafenib in human colon cancer cells. J Med Invest. 2015;62:195–8.
Jarroux J, Morillon A, Pinskaya M. History, Discovery, and Classification of lncRNAs. Adv Exp Med Biol. 2017;1008:1–46.
Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Molecular cell. 2011;43:904–14.
Li Y, Lu Y, Chen Y. Long non-coding RNA SNHG16 affects cell proliferation and predicts a poor prognosis in patients with colorectal cancer via sponging miR-200a-3p. Biosci Rep. 2019;39.
Engels BM, Hutvagner G. Principles and effects of microRNA-mediated post-transcriptional gene regulation. Oncogene. 2006;25:6163–9.
Volinia S, Galasso M, Costinean S, Tagliavini L, Gamberoni G, Drusco A, et al. Reprogramming of miRNA networks in cancer and leukemia. Genome Res. 2010;20:589–99.
Xi ZW, Xin SY, Zhou LQ, Yuan HX, Wang Q, Chen KX. Downregulation of rho-associated protein kinase 1 by miR-124 in colorectal cancer. World J Gastroenterol. 2015;21:5454–64.
Mestdagt M, Polette M, Buttice G, Noel A, Ueda A, Foidart JM, et al. Transactivation of MCP-1/CCL2 by beta-catenin/TCF-4 in human breast cancer cells. Int J Cancer. 2006;118:35–42.
Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–5.
Yoshidome H, Kohno H, Shida T, Kimura F, Shimizu H, Ohtsuka M, et al. Significance of monocyte chemoattractant protein-1 in angiogenesis and survival in colorectal liver metastases. Int J Oncol. 2009;34:923–30.
McClellan JL, Davis JM, Steiner JL, Enos RT, Jung SH, Carson JA, et al. Linking tumor-associated macrophages, inflammation, and intestinal tumorigenesis: role of MCP-1. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1087–95.
Zhao L, Lim SY, Gordon-Weeks AN, Tapmeier TT, Im JH, Cao Y, et al. Recruitment of a myeloid cell subset (CD11b/Gr1 mid) via CCL2/CCR2 promotes the development of colorectal cancer liver metastasis. Hepatology. 2013;57:829–39.
Kawano S, Nakamachi Y. miR-124a as a key regulator of proliferation and MCP-1 secretion in synoviocytes from patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70:i88–91.
Christensen LL, True K, Hamilton MP, Nielsen MM, Damas ND, Damgaard CK, et al. SNHG16 is regulated by the Wnt pathway in colorectal cancer and affects genes involved in lipid metabolism. Mol Oncol. 2016;10:1266–82.
Xu FF, Zha GQ, Wu YP, Cai WL, Ao J. Overexpressing lncRNA SNHG16 inhibited HCC proliferation and chemoresistance by functionally sponging hsa-miR-93. Onco Targets Ther. 2018;11:8855–63.
Cai C, Huo Q, Wang XL, Chen B, Yang QF. SNHG16 contributes to breast cancer cell migration by competitively binding miR-98 with E2F5. Biochem Bioph Res Co. 2017;485:272–8.
Wang X, Kan J, Han J, Zhang W, Bai L, Wu H. LncRNA SNHG16 Functions as an Oncogene by Sponging MiR-135a and Promotes JAK2/STAT3 Signal Pathway in Gastric Cancer. J Cancer. 2019;10:1013–22.
Lu YF, Cai XL, Li ZZ, Lv J, Xiang YA, Chen JJ, et al. LncRNA SNHG16 Functions as an Oncogene by Sponging MiR-4518 and Up-Regulating PRMT5 Expression in Glioma. Cell Physiol Biochem. 2018;45:1975–85.
Zhang K, Chen J, Song HZ, Chen LB. SNHG16/miR-140-5p axis promotes esophagus cancer cell proliferation, migration and EMT formation through regulating ZEB1. Oncotarget. 2018;9:1028–40.
Furukawa S, Soeda S, Kiko Y, Suzuki O, Hashimoto Y, Watanabe T, et al. MCP-1 promotes invasion and adhesion of human ovarian cancer cells. Anticancer Res. 2013;33:4785–90.
Yang C, Chen D, Huang K, Zhang H, Xu D, Tian Y, et al. The expression of chemokine MCP-1 in colorectal carcinoma and its relationship to the infiltration of macrophage. Chin German. J Clin Oncol. 2006;5:343–6.
Funding
This work was supported by Hunan provincial Natural Scientific Foundation [grant number 2017JJ3177] and National Natural Scientific Foundation of China [grant number 81502114].
Author information
Authors and Affiliations
Contributions
Z-YC and YP: guarantor of integrity of the entire study; Z-YC: study concepts, study design, manuscript preparation; X-YW: definition of intellectual content; Y-MY: literature research, manuscript editing; HC: clinical studies; YP: experimental studies, manuscript review; LY: data acquisition; M-HW: data analysis; D-TJ: statistical analysis.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Chen, ZY., Wang, XY., Yang, YM. et al. LncRNA SNHG16 promotes colorectal cancer cell proliferation, migration, and epithelial–mesenchymal transition through miR-124-3p/MCP-1. Gene Ther 29, 193–205 (2022). https://doi.org/10.1038/s41434-020-0176-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41434-020-0176-2
This article is cited by
-
Development and validation of cuproptosis-related lncRNA signatures for prognosis prediction in colorectal cancer
BMC Medical Genomics (2023)
-
Silencing LncRNA SNHG16 suppresses the diabetic inflammatory response by targeting the miR-212-3p/NF-κB signaling pathway
Diabetology & Metabolic Syndrome (2023)
-
Long non-coding RNA AC087388.1 as a novel biomarker in colorectal cancer
BMC Cancer (2022)
-
Long non-coding RNAs as the critical regulators of epithelial mesenchymal transition in colorectal tumor cells: an overview
Cancer Cell International (2022)
-
Integrated whole transcriptome and small RNA analysis revealed multiple regulatory networks in colorectal cancer
Scientific Reports (2021)