Abstract
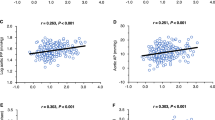
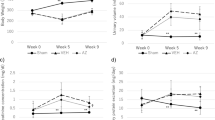
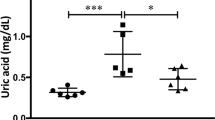
Circadian fluctuation disorder of the intrarenal renin–angiotensin system (RAS) causes that of blood pressure (BP) and renal damage. In renal damage with an impaired glomerular filtration barrier, liver-derived angiotensinogen (AGT) filtered through damaged glomeruli regulates intrarenal RAS activity. Furthermore, glomerular permeability is more strongly affected by glomerular hypertension than by systemic hypertension. Thus, we aimed to clarify whether the circadian rhythm of intrarenal RAS activity is influenced by AGT filtered through damaged glomeruli due to glomerular capillary pressure. Rats with adriamycin nephropathy and an impaired glomerular filtration barrier were compared with control rats. In adriamycin nephropathy rats, olmesartan medoxomil (an angiotensin II type 1 receptor blocker) or hydralazine (a vasodilator) was administered, and the levels of intrarenal RAS components in the active and rest phases were evaluated. Moreover, the diameter ratio of afferent to efferent arterioles (A/E ratio), an indicator of glomerular capillary pressure, and the glomerular sieving coefficient (GSC) based on multiphoton microscopy in vivo imaging, which reflects glomerular permeability, were determined. Mild renal dysfunction was induced, and the systemic BP increased, resulting in increased A/E ratios in the adriamycin nephropathy rats compared with the control rats. Fluctuations in intrarenal RAS activity occurred in parallel with circadian fluctuations in glomerular capillary pressure, which disappeared with olmesartan treatment and were maintained with hydralazine treatment. Furthermore, the GSCs for AGT also showed similar changes. In conclusion, intrarenal RAS activity is influenced by the filtration of liver-derived AGT from damaged glomeruli due to circadian fluctuation disorder of the glomerular capillary pressure.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Nakano D, Kobori H, Burford JL, Gevorgyan H, Seidel S, Hitomi H, et al. Multiphoton imaging of the glomerular permeability of angiotensinogen. J Am Soc Nephrol. 2012;23:1847–56.
Matsusaka T, Niimura F, Shimizu A, Pastan I, Saito A, Kobori H, et al. Liver angiotensinogen is the primary source of renal angiotensin II. J Am Soc Nephrol. 2012;23:1181–9.
Gordon RD, Wolfe LK, Island DP, Liddle GW. A diurnal rhythm in plasma renin activity in man. J Clin Invest. 1966;45:1587–92.
Kala R, Fyhrquist F, Eisalo A. Diurnal variation of plasma angiotensin II in man. Scand J Clin Lab Invest. 1973;31:363–5.
Hilfenhaus M. Circadian rhythm of the renin-angiotensin-aldosterone system in the rat. Arch Toxicol. 1976;36:305–16.
Isobe S, Ohashi N, Fujikura T, Tsuji T, Sakao Y, Yasuda H, et al. Disturbed circadian rhythm of the intrarenal renin-angiotensin system: relevant to nocturnal hypertension and renal damage. Clin Exp Nephrol. 2015;19:231–9.
Isobe S, Ohashi N, Ishigaki S, Tsuji T, Sakao Y, Kato A, et al. Augmented circadian rhythm of the intrarenal renin-angiotensin systems in anti-thymocyte serum nephritis rats. Hypertens Res. 2016;39:312–20.
Ohashi N, Isobe S, Ishigaki S, Yasuda H. Circadian rhythm of blood pressure and the renin-angiotensin system in the kidney. Hypertens Res. 2017;40:413–22.
Ohashi N, Ishigaki S, Isobe S. The pivotal role of melatonin in ameliorating chronic kidney disease by suppression of the renin-angiotensin system in the kidney. Hypertens Res. 2019;42:761–8.
Nishijima Y, Kobori H, Kaifu K, Mizushige T, Hara T, Nishiyama A, et al. Circadian rhythm of plasma and urinary angiotensinogen in healthy volunteers and in patients with chronic kidney disease. J Renin Angiotensin Aldosterone Syst. 2014;15:505–8.
Olson JL, Hostetter TH, Rennke HG, Brenner BM, Venkatachalam MA. Altered glomerular permselectivity and progressive sclerosis following extreme ablation of renal mass. Kidney Int. 1982;22:112–26.
Anderson S, Meyer TW, Rennke HG, Brenner BM. Control of glomerular hypertension limits glomerular injury in rats with reduced renal mass. J Clin Invest. 1985;76:612–9.
Griffin KA, Picken MM, Bidani AK. Deleterious effects of calcium channel blockade on pressure transmission and glomerular injury in rat remnant kidneys. J Clin Invest. 1995;96:793–800.
Sandoval RM, Molitoris BA, Palygin O. Fluorescent imaging and microscopy for dynamic processes in rats. Methods Mol Biol. 2019;2018:151–75.
Satoh M, Kobayashi S, Kuwabara A, Tomita N, Sasaki T, Kashihara N. In vivo visualization of glomerular microcirculation and hyperfiltration in streptozotocin-induced diabetic rats. Microcirculation. 2010;17:103–12.
Sandoval RM, Wagner MC, Patel M, Campos-Bilderback SB, Rhodes GJ, Wang E, et al. Multiple factors influence glomerular albumin permeability in rats. J Am Soc Nephrol. 2012;23:447–57.
Nakano D, Nishiyama A. Multiphoton imaging of kidney pathophysiology. J Pharm Sci. 2016;132:1–5.
Okuda S, Oh Y, Tsuruda H, Onoyama K, Fujimi S, Fujishima M. Adriamycin-induced nephropathy as a model of chronic progressive glomerular disease. Kidney Int. 1986;29:502–10.
Lee VW, Harris DC. Adriamycin nephropathy: a model of focal segmental glomerulosclerosis. Nephrology. 2011;16:30–8.
Hrenak J, Arendasova K, Rajkovicova R, Aziriova S, Repova K, Krajcirovicova K, et al. Protective effect of captopril, olmesartan, melatonin and compound 21 on doxorubicin-induced nephrotoxicity in rats. Physiol Res. 2013;62 Suppl 1:S181–9.
Huang Y, Yamamoto T, Misaki T, Suzuki H, Togawa A, Ohashi N, et al. Enhanced intrarenal receptor-mediated prorenin activation in chronic progressive anti-thymocyte serum nephritis rats on high salt intake. Am J Physiol Ren Physiol. 2012;303:F130–8.
Hosgood SA, Mohamed IH, Nicholson ML. The two layer method does not improve the preservation of porcine kidneys. Med Sci Monit. 2011;17:BR27–33.
Kobori H, Harrison-Bernard LM, Navar LG. Urinary excretion of angiotensinogen reflects intrarenal angiotensinogen production. Kidney Int. 2002;61:579–85.
Yamamoto T, Nakagawa T, Suzuki H, Ohashi N, Fukasawa H, Fujigaki Y, et al. Urinary angiotensinogen as a marker of intrarenal angiotensin II activity associated with deterioration of renal function in patients with chronic kidney disease. J Am Soc Nephrol. 2007;18:1558–65.
Kobori H, Navar LG. Urinary angiotensinogen as a novel biomarker of intrarenal renin-angiotensin system in chronic kidney disease. Int Rev Thromb. 2011;6:108–16.
Ohashi N, Yamamoto T, Huang Y, Misaki T, Fukasawa H, Suzuki H, et al. Intrarenal RAS activity and urinary angiotensinogen excretion in anti-thymocyte serum nephritis rats. Am J Physiol Ren Physiol. 2008;295:F1512–8.
Ohashi N, Isobe S, Ishigaki S, Suzuki T, Ono M, Fujikura T, et al. Intrarenal renin-angiotensin system activity is augmented after initiation of dialysis. Hypertens Res. 2017;40:364–70.
Ishigaki S, Ohashi N, Matsuyama T, Isobe S, Tsuji N, Iwakura T, et al. Melatonin ameliorates intrarenal renin-angiotensin system in a 5/6 nephrectomy rat model. Clin Exp Nephrol. 2018;22:539–49.
Ganten D, Wagner J, Zeh K, Bader M, Michel JB, Paul M, et al. Species specificity of renin kinetics in transgenic rats harboring the human renin and angiotensinogen genes. Proc Natl Acad Sci U S A. 1992;89:7806–10.
Molitoris BA, Sandoval RM. Intravital multiphoton microscopy of dynamic renal processes. Am J Physiol Ren Physiol. 2005;288:F1084–9.
Rosivall L, Mirzahosseini S, Toma I, Sipos A, Peti-Peterdi J. Fluid flow in the juxtaglomerular interstitium visualized in vivo. Am J Physiol Ren Physiol. 2006;291:F1241–7.
Rhodes GJ. Surgical preparation of rats and mice for intravital microscopic imaging of abdominal organs. Methods. 2017;128:129–38.
Kidokoro K, Cherney DZI, Bozovic A, Nagasu H, Satoh M, Kanda E, et al. Evaluation of glomerular hemodynamic function by Empagliflozin in diabetic mice using in vivo imaging. Circulation. 2019;140:303–15.
Monu SR, Ren Y, Masjoan-Juncos JX, Kutskill K, Wang H, Kumar N, et al. Connecting tubule glomerular feedback mediates tubuloglomerular feedback resetting after unilateral nephrectomy. Am J Physiol Ren Physiol. 2018;315:F806–11.
Peti-Peterdi J. Independent two-photon measurements of albumin GSC give low values. Am J Physiol Ren Physiol. 2009;296:F1255–7.
Sandoval RM, Molitoris BA. Quantifying glomerular permeability of fluorescent macromolecules using 2-photon microscopy in Munich Wistar rats. J Vis Exp. 2013. https://doi.org/10.3791/50052.
Schiessl IM, Castrop H. Angiotensin II AT2 receptor activation attenuates AT1 receptor-induced increases in the glomerular filtration of albumin: a multiphoton microscopy study. Am J Physiol Ren Physiol. 2013;305:F1189–200.
Schiessl IM, Kattler V, Castrop H. In vivo visualization of the antialbuminuric effects of the angiotensin-converting enzyme inhibitor enalapril. J Pharm Exp Ther. 2015;353:299–306.
Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharm Rev. 2007;59:251–87.
Navar LG, Harrison-Bernard LM, Nishiyama A, Kobori H. Regulation of intrarenal angiotensin II in hypertension. Hypertension. 2002;39:316–22.
Wuerzner G, Firsov D, Bonny O. Circadian glomerular function: from physiology to molecular and therapeutical aspects. Nephrol Dial Transpl. 2014;29:1475–80.
Bidani AK, Griffin KA, Williamson G, Wang X, Loutzenhiser R. Protective importance of the myogenic response in the renal circulation. Hypertension. 2009;54:393–8.
Burke M, Pabbidi MR, Farley J, Roman RJ. Molecular mechanisms of renal blood flow autoregulation. Curr Vasc Pharm. 2014;12:845–58.
Carlstrom M, Wilcox CS, Arendshorst WJ. Renal autoregulation in health and disease. Physiol Rev. 2015;95:405–511.
Su Y, Foppen E, Zhang Z, Fliers E, Kalsbeek A. Effects of 6-meals-a-day feeding and 6-meals-a-day feeding combined with adrenalectomy on daily gene expression rhythms in rat epididymal white adipose tissue. Genes Cells. 2016;21:6–24.
Lew SW, Bosch JP. Effect of diet on creatinine clearance and excretion in young and elderly healthy subjects and in patients with renal disease. J Am Soc Nephrol. 1991;2:856–65.
Bankir L, Roussel R, Bouby N. Protein- and diabetes-induced glomerular hyperfiltration: role of glucagon, vasopressin, and urea. Am J Physiol Ren Physiol. 2015;309:F2–23.
Gabbai FB. The role of renal response to amino acid infusion and oral protein load in normal kidneys and kidney with acute and chronic disease. Curr Opin Nephrol Hypertens. 2018;27:23–9.
Satoh M, Haruna Y, Fujimoto S, Sasaki T, Kashihara N. Telmisartan improves endothelial dysfunction and renal autoregulation in Dahl salt-sensitive rats. Hypertens Res. 2010;33:135–42.
Tanner GA. Glomerular sieving coefficient of serum albumin in the rat: a two-photon microscopy study. Am J Physiol Ren Physiol. 2009;296:F1258–65.
Matsusaka T, Niimura F, Pastan I, Shintani A, Nishiyama A, Ichikawa I. Podocyte injury enhances filtration of liver-derived angiotensinogen and renal angiotensin II generation. Kidney Int. 2014;85:1068–77.
Acknowledgements
We acknowledge Dr Kengo Kidokoro (Kawasaki Medical School), Dr Daisuke Nakano (Kagawa University), Dr Chiharu Uchida (Hamamatsu University School of Medicine), and Dr Toshiyuki Ojima (Hamamatsu University School of Medicine) for critical technical support and important advice. We also acknowledge Daiichi Sankyo Co. (Tokyo, Japan) for providing olmesartan medoxomil (CS-866). This study was supported by a Grant-in-Aid for Scientific Research (17K09693 to NO) from the Ministry of Education, Culture, Sports, Science and Technology, Japan, and by grants from the Young Investigator Research Projects of Hamamatsu University School of Medicine in 2018 (awarded to TM).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Matsuyama, T., Ohashi, N., Aoki, T. et al. Circadian rhythm of the intrarenal renin–angiotensin system is caused by glomerular filtration of liver-derived angiotensinogen depending on glomerular capillary pressure in adriamycin nephropathy rats. Hypertens Res 44, 618–627 (2021). https://doi.org/10.1038/s41440-021-00620-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-021-00620-6
Keywords
This article is cited by
-
Emerging topics on basic research in hypertension: interorgan communication and the need for interresearcher collaboration
Hypertension Research (2023)
-
Update on Hypertension Research in 2021
Hypertension Research (2022)