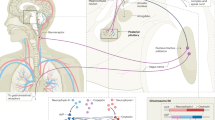
Abstract
Serum uric acid (UA) level is associated with the high cumulative incidence or prevalence of coronary artery disease (CAD), and hyperuricemia is considered as an independent risk marker for CAD. Sleep-disordered breathing (SDB) is also associated with an increased risk of CAD. Several studies have shown that SDB is associated with hyperuricemia, but the mechanisms are unclear. We measured serum levels of UA and xanthine oxidoreductase (XOR) activity and urinary levels of 8-hydroxy-2’-deoxyguanosine (8-OHdG), all of which were assessed at 6 p.m. and the following 6 a.m. in males with CAD. In addition, nocturnal pulse oximetry was performed for the night. Overall 32 eligible patients with CAD were enrolled. Serum UA levels significantly increased overnight. (5.32 ± 0.98 mg/dl to 5.46 ± 1.02 mg/dl, p < 0.001) Moreover, XOR activity and urinary 8-OHdG levels significantly increased from 6 p.m. to 6 a.m. Furthermore, 3% Oxygen desaturation index (ODI) was correlated with the overnight changes in XOR activity (r = 0.36, P = 0.047) and urinary 8-OHdG levels (r = 0.41, P = 0.02). In addition, 3%ODI was independently correlated with the changes in XOR activity (correlation coefficient, 0.36; P = 0.047) and 8-OHdG (partial correlation coefficient, 0.63; P = 0.004) in multivariable analyses. SDB severity was associated with the overnight changes in XOR activity and urinary 8-OHdG, suggesting that SDB may be associated with oxidative stress via UA production. This trial is registered at University Hospital Medical Information Network (UMIN), number: UMIN000021624.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Lee CH, Sethi R, Li R, Ho HH, Hein T, Jim MH, et al. Obstructive sleep apnea and cardiovascular events after percutaneous coronary intervention. Circulation. 2016;133:2008–17.
Peker Y, Hedner J, Kraiczi H, Löth S. Respiratory disturbance index: an independent predictor of mortality in coronary artery disease. Am J Respir Crit Care Med. 2000;162:81–6.
Mooe T, Franklin KA, Holmström K, Rabben T, Wiklund U. Sleep-disordered breathing and coronary artery disease: long-term prognosis. Am J Respir Crit Care Med. 2001;164:1910–3.
Yumino D, Tsurumi Y, Takagi A, Suzuki K, Kasanuki H. Impact of obstructive sleep apnea on clinical and angiographic outcomes following percutaneous coronary intervention in patients with acute coronary syndrome. Am J Cardiol. 2007;99:26–30.
Javaheri S, Barbe F, Campos-Rodriguez F, Dempsey JA, Khayat R, Javaheri S, et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol. 2017;69:841–58.
Floras JS. Sleep apnea and cardiovascular risk. J Cardiol. 2014;63:3–8.
Kasai T, Floras JS, Bradley TD. Sleep apnea and cardiovascular disease: a bidirectional relationship. Circulation. 2012;126:1495–510.
Linz D, Woehrle H, Bitter T, Fox H, Cowie MR, Böhm M, et al. The importance of sleep-disordered breathing in cardiovascular disease. Clin Res Cardiology: Off J Ger Card Soc. 2015;104:705–18.
Karimzadeh F, Nami M, Boostani R. Sleep microstructure dynamics and neurocognitive performance in obstructive sleep apnea syndrome patients. J Integr Neurosci. 2017;16:127–42.
Alvarez-Sabin J, Romero O, Delgado P, Quintana M, Santamarina E, Ferre A, et al. Obstructive sleep apnea and silent cerebral infarction in hypertensive individuals. J Sleep Res. 2018;27:232–9.
Li L, Lu J, Xue W, Wang L, Zhai Y, Fan Z, et al. Target of obstructive sleep apnea syndrome merge lung cancer: based on big data platform. Oncotarget. 2017;8:21567–78.
Micarelli A, Liguori C, Viziano A, Izzi F, Placidi F, Alessandrini M. Integrating postural and vestibular dimensions to depict impairment in moderate-to-severe obstructive sleep apnea syndrome patients. J Sleep Res. 2017;26:487–94.
Yatsu S, Naito R, Kasai T, Matsumoto H, Shitara J, Shimizu M, et al. Influence of sleep-disordered breathing assessed by pulse oximetry on long-term clinical outcomes in patients who underwent percutaneous coronary intervention. Clin Res cardiology: Off J Ger Card Soc. 2018;107:711–8.
Kuwabara M, Kanbay M, Hisatome I. Uric acid and hypertension because of arterial stiffness. Hypertension. 2018;72:582–4.
Liu Z, Chen T, Niu H, Ren W, Li X, Cui L, et al. The establishment and characteristics of rat model of atherosclerosis induced by hyperuricemia. Stem Cells Int. 2016;2016:1365257–7.
Chou YT, Chuang LP, Li HY, Fu JY, Lin SW, Yang CT, et al. Hyperlipidaemia in patients with sleep-related breathing disorders: prevalence & risk factors. Indian J Med Res. 2010;131:121–5.
Hira HS, Shukla A, Kaur A, Kapoor S. Serum uric acid and lactate levels among patients with obstructive sleep apnea syndrome: which is a better marker of hypoxemia? Ann Saudi Med. 2012;32:37–42.
Hirotsu C, Tufik S, Guindalini C, Mazzotti DR, Bittencourt LR, Andersen ML. Association between uric acid levels and obstructive sleep apnea syndrome in a large epidemiological sample. PloS One. 2013;8:e66891.
Grum CM, Simon RH, Dantzker DR, Fox IH. Evidence for adenosine triphosphate degradation in critically-ill patients. Chest. 1985;88:763–7.
Ruiz García A, Sánchez Armengol A, Luque Crespo García Aguilar D, Romero Falcón A, Carmona Bernal C, Capote F. Blood uric acid levels in patients with sleep-disordered breathing. Arch Bronconeumol. 2006;42:492–500.
Wysocka E, Cofta S, Piorunek T, Dziegielewska-Gesiak S, Bryl W, Torlinski L. Blood antioxidant status, dysglycemia and obstructive sleep apnea. Adv Exp Med Biol. 2013;756:121–9.
Hakoda M, Masunari N, Yamada M, Fujiwara S, Suzuki G, Kodama K, et al. Serum uric acid concentration as a risk factor for cardiovascular mortality: a longterm cohort study of atomic bomb survivors. J Rheumatol. 2005;32:906–12.
Murase T, Nampei M, Oka M, Miyachi A, Nakamura T. A highly sensitive assay of human plasma xanthine oxidoreductase activity using stable isotope-labeled xanthine and LC/TQMS. J Chromatogr B, Anal Technol Biomed Life Sci. 2016;1039:51–8.
Morrison B, Shenkin A, McLelland A, Robertson DA, Barrowman M, Graham S, et al. Intra-individual variation in commonly analyzed serum constituents. Clin Chem. 1979;25:1799–805.
Devgun MS, Dhillon HS. Importance of diurnal variations on clinical value and interpretation of serum urate measurements. J Clin Pathol. 1992;45:110–3.
Sennels HP, Jørgensen HL, Goetze JP, Fahrenkrug J. Rhythmic 24-hour variations of frequently used clinical biochemical parameters in healthy young males–the Bispebjerg study of diurnal variations. Scand J Clin Lab Investig. 2012;72:287–95.
Ozanturk E, Ucar ZZ, Varol Y, Koca H, Demir AU, Kalenci D, et al. Urinary uric acid excretion as an indicator of severe hypoxia and mortality in patients with obstructive sleep apnea and chronic obstructive pulmonary disease. Rev Port Pneumol (2006). 2016;22:18–26.
Kayyali US, Donaldson C, Huang H, Abdelnour R, Hassoun PM. Phosphorylation of xanthine dehydrogenase/oxidase in hypoxia. J Biol Chem. 2001;276:14359–65.
Berry CE, Hare JM. Xanthine oxidoreductase and cardiovascular disease: molecular mechanisms and pathophysiological implications. J Physiol. 2004;555:589–606.
Xu L, Yang Y, Chen J. The role of reactive oxygen species in cognitive impairment associated with sleep apnea. Exp Ther Med. 2020;20:4.
Pilkauskaite G, Miliauskas S, Sakalauskas R. Reactive oxygen species production in peripheral blood neutrophils of obstructive sleep apnea patients. ScientificWorldJournal. 2013;2013:421763.
Vatansever E, Surmen-Gur E, Ursavas A, Karadag M. Obstructive sleep apnea causes oxidative damage to plasma lipids and proteins and decreases adiponectin levels. Sleep Breath = Schlaf Atm. 2011;15:275–82.
Lavie L. Oxidative stress inflammation and endothelial dysfunction in obstructive sleep apnea. Front Biosci (Elite Ed). 2012;4:1391–403.
Yamauchi M, Nakano H, Maekawa J, Okamoto Y, Ohnishi Y, Suzuki T, et al. Oxidative stress in obstructive sleep apnea. Chest. 2005;127:1674–9.
Corte ED, Stirpe F. The regulation of rat liver xanthine oxidase. Involvement of thiol groups in the conversion of the enzyme activity from dehydrogenase (type D) into oxidase (type O) and purification of the enzyme. Biochem J. 1972;126:739–45.
Cantu-Medellin N, Kelley EE. Xanthine oxidoreductase-catalyzed reactive species generation: a process in critical need of reevaluation. Redox Biol. 2013;1:353–8.
Lira AB, de Sousa Rodrigues CF. Evaluation of oxidative stress markers in obstructive sleep apnea syndrome and additional antioxidant therapy: a review article. Sleep Breath = Schlaf Atm. 2016;20:1155–60.
Celec P, Jurkovičová I, Buchta R, Bartík I, Gardlík R, Pálffy R, et al. Antioxidant vitamins prevent oxidative and carbonyl stress in an animal model of obstructive sleep apnea. Sleep Breath = Schlaf Atm. 2013;17:867–71.
Cofta S, Winiarska HM, Płóciniczak A, Bielawska L, Brożek A, Piorunek T, et al. Oxidative stress markers and severity of obstructive sleep apnea. Adv Exp Med Biol. 2019;1222:27–35.
Bogardus C, Lillioja S, Mott D, Reaven GR, Kashiwagi A, Foley JE. Relationship between obesity and maximal insulin-stimulated glucose uptake in vivo and in vitro in Pima Indians. J Clin Investig. 1984;73:800–5.
Toyoki D, Shibata S, Kuribayashi-Okuma E, Xu N, Ishizawa K, Hosoyamada M, et al. Insulin stimulates uric acid reabsorption via regulating urate transporter 1 and ATP-binding cassette subfamily G member 2. Am J Physiol Ren Physiol. 2017;313:F826–34.
Tanaka Y, Nagoshi T, Takahashi H, Oi Y, Yoshii A, Kimura H, et al. URAT1-selective inhibition ameliorates insulin resistance by attenuating diet-induced hepatic steatosis and brown adipose tissue whitening in mice. Mol Metab. 2022;55:101411.
Pan XF, Wang L, Pan A. Epidemiology and determinants of obesity in China. lancet Diabetes Endocrinol. 2021;9:373–92.
GBD Obesity C, Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, et al. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl J Med. 2017;377:13–27.
Chaiamnuay P, Darmawan J, Muirden KD, Assawatanabodee P. Epidemiology of rheumatic disease in rural Thailand: a WHO-ILAR COPCORD study. Community Oriented Programme for the Control of Rheumatic Disease. J Rheumatol. 1998;25:1382–7.
Kuo CF, Grainge MJ, See LC, Yu KH, Luo SF, Zhang W, et al. Epidemiology and management of gout in Taiwan: a nationwide population study. Arthritis Res Ther. 2015;17:13.
Lam B, Ip MS, Tench E, Ryan CF. Craniofacial profile in Asian and white subjects with obstructive sleep apnoea. Thorax. 2005;60:504–10.
Matsuo H, Takada T, Ichida K, Nakamura T, Nakayama A, Ikebuchi Y, et al. Common defects of ABCG2, a high-capacity urate exporter, cause gout: a function-based genetic analysis in a Japanese population. Sci Transl Med. 2009;1:5ra11.
Acknowledgements
We thanks the patients and all staff in Juntendo University Hospital for their important contributions.
Funding
This study was funded by Sanwa Kagaku Kenkyusho Co.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
TK received research funds from Sanwa Kagaku Kenkyusho Co. and ResMed. AS and RN are affiliated with a department endowed by Philips, ResMed, and Fukuda Denshi. HD reports honoraria from Amgen Astellas, Daiichi Sankyo, Kowa, MSD, and research grant from Canon, and scholarship grant from Otsuka, Sanofi, MSD, Daiichi Sankyo, Pfizer, Mitsubishi Tanabe, Astellas, Takeda, Teijin, Shionogi, Actelion, Kowa, Bayer, Boehringer Ingelheim, and courses endowed by Phillips, Resmed, Fukuda Denshi, Asahikasei, Inter-Reha, and Toho Holdings. Takayo Murase and Takashi Nakamura are employed by Sanwa Kagaku Kenkyusho Co. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shimizu, M., Kasai, T., Naito, R. et al. Overnight changes in uric acid, xanthine oxidoreductase and oxidative stress levels and their relationships with sleep-disordered breathing in patients with coronary artery disease. Hypertens Res 46, 2293–2301 (2023). https://doi.org/10.1038/s41440-023-01331-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-023-01331-w
Keywords
This article is cited by
-
Can we obtain a reliable marker that shows the hypoxic burden in patients with sleep disordered breathing?
Hypertension Research (2023)
-
How do we tackle nighttime blood pressure?
Hypertension Research (2023)