Abstract
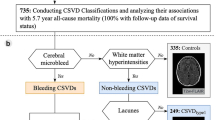
To investigate the association between vascular risk factors and progression of cerebral small vessel disease (SVD), we conducted a longitudinal study with neurologically healthy cohort composed mostly of middle-aged adults (n = 665, mean age, 57.7 years). Subjects, who had both baseline data of brain health examinations including MRI and follow-up MRI at least 1 year after the baseline MRI, were included this study. The presence of features of SVD, including lacunes, cerebral microbleeds, white matter hyperintensity, and basal ganglia perivascular spaces were summed to obtain “total SVD score” (range, 0–4). Progression of SVD was evaluated among subjects with a total SVD score of ≤ 3 and was defined as a ≥ 1 point increase in that score at follow-up relative to baseline. As the primary analysis, multivariate logistic regression analyses were performed to determine the associations of progression of SVD at baseline. The median follow-up period was 7.3 years and progression of SVD was observed in 154 subjects (23.2%). Even after adjustment with confounders multivariate logistic regression analyses showed that progression of SVD was associated with age (per 10-year increase, odds ratio [OR]: 2.08, 95% confidence interval [CI] 1.62–2.67), hypertension (OR 1.55, 95%CI 1.05–2.29), systolic blood pressure (BP) (per standard deviation [SD] increase, OR 1.27, 95%CI 1.04–1.54), diastolic BP (per SD increase, OR 1.23, 95%CI 1.01–1.50), and mean arterial pressure (per SD increase, OR 1.27, 95%CI 1.04–1.55). Age and high blood pressure appear to play key roles in the progression of cerebral small vessel burden after mid-life.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701.
Gouw AA, Seewann A, van der Flier WM, Barkhof F, Rozemuller AM, Scheltens P, et al. Heterogeneity of small vessel disease: a systematic review of MRI and histopathology correlations. J Neurol Neurosurg Psychiatry. 2011;82:126–35.
Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–38.
Yakushiji Y. Cerebral microbleeds: detection, associations and clinical implications. Front Neurol Neurosci. 2015;37:78–92.
Klarenbeek P, van Oostenbrugge RJ, Rouhl RP, Knottnerus IL, Staals J. Ambulatory blood pressure in patients with lacunar stroke: association with total MRI burden of cerebral small vessel disease. Stroke. 2013;44:2995–9.
Staals J, Makin SD, Doubal FN, Dennis MS, Wardlaw JM. Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology. 2014;83:1228–34.
Staals J, Booth T, Morris Z, Bastin ME, Gow AJ, Corley J, et al. Total MRI load of cerebral small vessel disease and cognitive ability in older people. Neurobiol Aging. 2015;36:2806–11.
Suzuyama K, Yakushiji Y, Ogata A, Nishihara M, Eriguchi M, Kawaguchi A, et al. Total small vessel disease score and cerebro-cardiovascular events in healthy adults: The Kashima scan study. Int J Stroke. 2020;15:973–9.
Yakushiji Y, Charidimou A, Hara M, Noguchi T, Nishihara M, Eriguchi M, et al. Topography and associations of perivascular spaces in healthy adults: the Kashima scan study. Neurology. 2014;83:2116–23.
Yakushiji Y, Noguchi T, Charidimou A, Eriguchi M, Nishihara M, Hara M, et al. Basal ganglia cerebral microbleeds and global cognitive function: the Kashima Scan Study. J Stroke Cerebrovasc Dis. 2015;24:431–9.
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–7.
Yakushiji Y, Charidimou A, Noguchi T, Nishihara M, Eriguchi M, Nanri Y, et al. Total Small Vessel Disease Score in Neurologically Healthy Japanese Adults in the Kashima Scan Study. Intern Med. 2018;57:189–96.
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98.
Flostein MF, Flostein SE, Franjiang G. Mini-mental state examination: clinical guide. Psychological Assessment Resources, Inc, Lutz, FL, 2001.
Yakushiji Y, Horikawa E, Eriguchi M, Nanri Y, Nishihara M, Hirotsu T, et al. Norms of the Mini-Mental State Examination for Japanese subjects that underwent comprehensive brain examinations: the Kashima Scan Study. Intern Med. 2014;53:2447–53.
Yakushiji Y, Nishiyama M, Yakushiji S, Hirotsu T, Uchino A, Nakajima J, et al. Brain microbleeds and global cognitive function in adults without neurological disorder. Stroke. 2008;39:3323–8.
Henskens LH, van Oostenbrugge RJ, Kroon AA, de Leeuw PW, Lodder J. Brain microbleeds are associated with ambulatory blood pressure levels in a hypertensive population. Hypertension. 2008;51:62–68.
Hara M, Yakushiji Y, Nannri H, Sasaki S, Noguchi T, Nishiyama M, et al. Joint effect of hypertension and lifestyle-related risk factors on the risk of brain microbleeds in healthy individuals. Hypertens Res. 2013;36:789–94.
Hara M, Yakushiji Y, Suzuyama K, Nishihara M, Eriguchi M, Noguchi T, et al. Synergistic effect of hypertension and smoking on the total small vessel disease score in healthy individuals: the Kashima scan study. Hypertens Res. 2019;42:1738–44.
Bleakley C, Hamilton PK, Pumb R, Harbinson M, McVeigh GE. Endothelial function in hypertension: victim or culprit? J Clin Hypertens. (Greenwich). 2015;17:651–4.
Mazzone A, Cusa C, Mazzucchelli I, Vezzoli M, Ottini E, Ghio S, et al. Cigarette smoking and hypertension influence nitric oxide release and plasma levels of adhesion molecules. Clin Chem Lab Med. 2001;39:822–6.
Leone A. Smoking and hypertension: independent or additive effects to determining vascular damage? Curr Vasc Pharmacol. 2011;9:585–93.
Kulshreshtha A, Vaccarino V, Judd SE, Howard VJ, McClellan WM, Muntner P, et al. Life’s Simple 7 and risk of incident stroke: the reasons for geographic and racial differences in stroke study. Stroke. 2013;44:1909–14.
Samieri C, Perier MC, Gaye B, Proust-Lima C, Helmer C, Dartigues JF, et al. Association of cardiovascular health level in older age with cognitive decline and incident dementia. JAMA. 2018;320:657–64.
Seliger SL, Longstreth WT Jr. Lessons about brain vascular disease from another pulsating organ, the kidney. Stroke. 2008;39:5–6.
Poels MM, Ikram MA, van der Lugt A, Hofman A, Krestin GP, Breteler MM, et al. Incidence of cerebral microbleeds in the general population: the Rotterdam Scan Study. Stroke. 2011;42:656–61.
Schmidt R, Fazekas F, Kapeller P, Schmidt H, Hartung HP. MRI white matter hyperintensities: three-year follow-up of the Austrian Stroke Prevention Study. Neurology. 1999;53:132–9.
Tendolkar I, Enajat M, Zwiers MP, van Wingen G, de Leeuw FE, van Kuilenburg J, et al. One-year cholesterol lowering treatment reduces medial temporal lobe atrophy and memory decline in stroke-free elderly with atrial fibrillation: evidence from a parallel group randomized trial. Int J Geriatr Psychiatry. 2012;27:49–58.
Winkleby MA, Kraemer HC, Ahn DK, Varady AN. Ethnic and socioeconomic differences in cardiovascular disease risk factors: findings for women from the Third National Health and Nutrition Examination Survey, 1988-1994. JAMA. 1998;280:356–62.
Yakushiji Y, Wilson D, Ambler G, Charidimou A, Beiser A, van Buchem MA, et al. Distribution of cerebral microbleeds in the East and West: Individual participant meta-analysis. Neurology. 2019;92:e1086–97.
van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9:167–76.
Yakushiji Y, Tanaka J, Wilson D, Charidimou A, Noguchi T, Kawashima M, et al. Proportion of intracerebral haemorrhage due to cerebral amyloid angiopathy in the East and West: Comparison between single hospital centres in Japan and the United Kingdom. J Neurol Sci. 2020;416:117037.
Funding
This study was supported by a Grant-in-Aid for Scientific Research (C), JSPS KAKENHI (Grant No. 21K10510).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The following conflict of interest is outside the submitted work. Yakushiji reports personal fees from Daiichi-Sankyo. The other authors report no conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ide, T., Yakushiji, Y., Suzuyama, K. et al. Associations for progression of cerebral small vessel disease burden in healthy adults: the Kashima scan study. Hypertens Res 47, 302–310 (2024). https://doi.org/10.1038/s41440-023-01419-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-023-01419-3
Keywords
This article is cited by
-
Intriguing review and topics in this month of Hypertension Research
Hypertension Research (2024)