Abstract
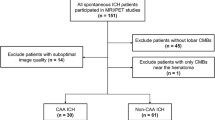
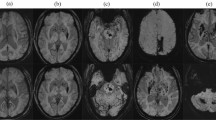
Primary aldosteronism is associated with various types of cardiovascular and cerebrovascular damage independently of hypertension. Although chronic hypertension and related cerebral arteriosclerosis are the main risk factors for intracerebral hemorrhage, the effects of aldosteronism remain poorly understood. We enrolled 90 survivors of hypertensive intracerebral hemorrhage, 21 of them with aldosteronism and 69 with essential hypertension as controls in this study. Clinical parameters and neuroimaging markers of cerebral small vessel disease were recorded, and its correlations with aldosteronism were investigated. Our results showed that the aldosteronism group (55.2 ± 9.7 years, male 47.6%) had similar hypertension severity but exhibited a higher cerebral microbleed count (interquartile range) (8.5 [2.0‒25.8] vs 3 [1.0‒6.0], P = 0.005) and higher severity of dilated perivascular space in the basal ganglia (severe perivascular space [number >20], 52.4% vs. 24.6%, P = 0.029; large perivascular space [>3 mm], 52.4% vs. 20.3%, P = 0.010), compared to those with essential hypertension (53.8 ± 11.7 years, male 73.9%). In multivariate models, aldosteronism remained an independent predictor of a higher (>10) microbleed count (odds ratio = 8.60, P = 0.004), severe perivascular space (odds ratio = 4.00, P = 0.038); the aldosterone-to-renin ratio was associated with dilated perivascular space (P = 0.043) and large perivascular space (P = 0.008). In conclusions, survivors of intracerebral hemorrhage with aldosteronism showed a tendency towards more severe hypertensive arteriopathy than the essential hypertension counterparts independently of blood pressure; aldosteronism may contribute to dilated perivascular space around the deep perforating arteries.

Aldosteronism is associated with more severe cerebral small vessel disease in hypertensive intracerebral hemorrhage.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Rossi GP, Bernini G, Caliumi C, Desideri G, Fabris B, Ferri C, et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. 2006;48:2293–2300.
Douma S, Petidis K, Doumas M, Papaefthimiou P, Triantafyllou A, Kartali N, et al. Prevalence of primary hyperaldosteronism in resistant hypertension: a retrospective observational study. Lancet. 2008;371:1921–6.
Strauch B, Petrak O, Zelinka T, Wichterle D, Holaj R, Kasalicky M, et al. Adrenalectomy improves arterial stiffness in primary aldosteronism. Am J hypertens. 2008;21:1086–92.
Lin YH, Lin LY, Chen A, Wu XM, Lee JK, Su TC, et al. Adrenalectomy improves increased carotid intima-media thickness and arterial stiffness in patients with aldosterone producing adenoma. Atherosclerosis. 2012;221:154–9.
Lin YH, Huang KH, Lee JK, Wang SM, Yen RF, Wu VC, et al. Factors influencing left ventricular mass regression in patients with primary aldosteronism post adrenalectomy. J Renin Angiotensin Aldosterone Syst. 2011;12:48–53.
Weber KT, Brilla CG, Janicki JS, Reddy HK, Campbell SE. Myocardial fibrosis: role of ventricular systolic pressure, arterial hypertension, and circulating hormones. Basic Res Cardiol. 1991;86:25–31.
Rossi GP, Di Bello V, Ganzaroli C, Sacchetto A, Cesari M, Bertini A, et al. Excess aldosterone is associated with alterations of myocardial texture in primary aldosteronism. Hypertension. 2002;40:23–27.
Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45:1243–8.
Holaj R, Zelinka T, Wichterle D, Petrak O, Strauch B, Widimsky J Jr. Increased intima-media thickness of the common carotid artery in primary aldosteronism in comparison with essential hypertension. J hypertens. 2007;25:1451–7.
Yuan Y, Li N, Liu Y, Wang M, Heizhati M, Zhu Q, et al. Plasma aldosterone concentration is associated with white matter lesions in patients with primary aldosteronism. Endocrine.2022;75:889–98.
Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: a retrospective cohort study. Lancet Diabetes Endocrinol. 2018;6:51–59.
Ohno Y, Sone M, Inagaki N, Yamasaki T, Ogawa O, Takeda Y, et al. Prevalence of cardiovascular disease and its risk factors in primary aldosteronism: a multicenter study in Japan. Hypertension. 2018;71:530–7.
Chang YH, Chung SD, Wu CH, Chueh JS, Chen L, Lin PC, et al. Surgery decreases the long-term incident stroke risk in patients with primary aldosteronism. Surgery. 2020;167:367–77.
Mulatero P, Monticone S, Bertello C, Viola A, Tizzani D, Iannaccone A, et al. Long-term cardio- and cerebrovascular events in patients with primary aldosteronism. J Clin Endocrinol Metab. 2013;98:4826–33.
Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N. Engl J Med. 2001;344:1450–60.
Wardlaw JM, Smith C, Dichgans M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol. 2019;18:684–96.
McCarthy J, Yang J, Clissold B, Young MJ, Fuller PJ, Phan T. Hypertension management in stroke prevention: time to consider primary aldosteronism. Stroke. 2021;52:e626–34.
Tsai HH, Chen SJ, Tsai LK, Pasi M, Lo YL, Chen YF, et al. Long-Term vascular outcomes in patients with mixed location intracerebral hemorrhage and microbleeds. Neurology. 2021;96:e995–1004.
Yeh SJ, Tang SC, Tsai LK, Jeng JS. Pathogenetical subtypes of recurrent intracerebral hemorrhage: designations by SMASH-U classification system. Stroke. 2014;45:2636–42.
Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J. 1949;37:161–86.
Casale PN, Devereux RB, Alonso DR, Campo E, Kligfield P. Improved sex-specific criteria of left ventricular hypertrophy for clinical and computer interpretation of electrocardiograms: validation with autopsy findings. Circulation. 1987;75:565–72.
Wu VC, Hu YH, Er LK, Yen RF, Chang CH, Chang YL, et al. Case detection and diagnosis of primary aldosteronism - The consensus of Taiwan Society of Aldosteronism. J Formos Med Assoc. 2017;116:993–1005.
Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–38.
Gregoire SM, Chaudhary UJ, Brown MM, Yousry TA, Kallis C, Jager HR, et al. The Microbleed Anatomical Rating Scale (MARS): reliability of a tool to map brain microbleeds. Neurology. 2009;73:1759–66.
Pasi M, Boulouis G, Fotiadis P, Auriel E, Charidimou A, Haley K, et al. Distribution of lacunes in cerebral amyloid angiopathy and hypertensive small vessel disease. Neurology. 2017;88:2162–8.
Tsai HH, Pasi M, Tsai LK, Chen YF, Chen YW, Tang SC, et al. Superficial cerebellar microbleeds and cerebral amyloid angiopathy: a magnetic resonance imaging/positron emission tomography study. Stroke. 2020;51:202–8.
Tsai HH, Pasi M, Tsai LK, Chen YF, Lee BC, Tang SC, et al. Microangiopathy underlying mixed-location intracerebral hemorrhages/microbleeds: a PiB-PET study. Neurology. 2019;92:e774–81.
Charidimou A, Boulouis G, Pasi M, Auriel E, van Etten ES, Haley K, et al. MRI-visible perivascular spaces in cerebral amyloid angiopathy and hypertensive arteriopathy. Neurology. 2017;88:1157–64.
Doubal FN, MacLullich AM, Ferguson KJ, Dennis MS, Wardlaw JM. Enlarged perivascular spaces on MRI are a feature of cerebral small vessel disease. Stroke. 2010;41:450–4.
Ding J, Sigurethsson S, Jonsson PV, Eiriksdottir G, Charidimou A, Lopez OL, et al. Large perivascular spaces visible on magnetic resonance imaging, cerebral small vessel disease progression, and risk of dementia: the age, gene/environment susceptibility-reykjavik study. JAMA Neurol. 2017;74:1105–12.
Chen ZW, Tsai CH, Pan CT, Chou CH, Liao CW, Hung CS, et al. Endothelial dysfunction in primary aldosteronism. Int J Mol Sci. 2019;20:5214.
Dinh QN, Young MJ, Evans MA, Drummond GR, Sobey CG, Chrissobolis S. Aldosterone-induced oxidative stress and inflammation in the brain are mediated by the endothelial cell mineralocorticoid receptor. Brain Res. 2016;1637:146–53.
Linn J, Halpin A, Demaerel P, Ruhland J, Giese AD, Dichgans M, et al. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology. 2010;74:1346–50.
Tsai HH, Kim JS, Jouvent E, Gurol ME. Updates on prevention of hemorrhagic and lacunar strokes. J Stroke. 2018;20:167–79.
Cuadrado-Godia E, Dwivedi P, Sharma S, Ois Santiago A, Roquer Gonzalez J, Balcells M, et al. Cerebral small vessel disease: a review focusing on pathophysiology, biomarkers, and machine learning strategies. J Stroke. 2018;20:302–20.
Pasi M, Charidimou A, Boulouis G, Auriel E, Ayres A, Schwab KM, et al. Mixed-location cerebral hemorrhage/microbleeds: underlying microangiopathy and recurrence risk. Neurology. 2018;90:e119–26.
Blanc C, Viguier A, Calviere L, Planton M, Albucher JF, Rousseau V, et al. Underlying small vessel disease associated with mixed cerebral microbleeds. Front Neurol. 2019;10:1126.
Wardlaw JM, Benveniste H, Nedergaard M, Zlokovic BV, Mestre H, Lee H, et al. Perivascular spaces in the brain: anatomy, physiology and pathology. Nat Rev Neurol. 2020;16:137–53.
Zhu YC, Tzourio C, Soumare A, Mazoyer B, Dufouil C, Chabriat H. Severity of dilated Virchow-Robin spaces is associated with age, blood pressure, and MRI markers of small vessel disease: a population-based study. Stroke. 2010;41:2483–90.
Weber KT. Aldosteronism revisited: perspectives on less well-recognized actions of aldosterone. J Lab Clin Med. 2003;142:71–82.
Weber KT, Singh KD, Hey JC. Idiopathic intracranial hypertension with primary aldosteronism: report of 2 cases. Am J Med Sci. 2002;324:45–50.
Riba-Llena I, Jimenez-Balado J, Castane X, Girona A, Lopez-Rueda A, Mundet X, et al. Arterial stiffness is associated with basal ganglia enlarged perivascular spaces and cerebral small vessel disease load. Stroke. 2018;49:1279–81.
Kim BJ, Kim JS. Ischemic stroke subtype classification: an asian viewpoint. J Stroke. 2014;16:8–17.
TAIPAI study group
Vin-Cent Wu12, Tai-Shuan Lai12, Shih-Chieh Jeff Chueh12, Shao-Yu Yang12, Kao-Lang Liu12, Chin-Chen Chang12, Bo-Ching Lee12, Shuo-Meng Wang12, Kuo-How Huang12, Po-Chih Lin12, Yen-Hung Lin12, Chi-Sheng Hung12, Lian-Yu Lin12, Shih-Cheng Liao12, Ching-Chu Lu12, Chieh-Kai Chan12, Leay-Kiaw Er13, Ya-Hui Hu13, Che-Hsiung Wu13, Yao-Chou Tsai13, Zheng-Wei Chen14, Chien-Ting Pan14, Che-Wei Liao10, Cheng-Hsuan Tsai15, Yi-Yao Chang16, Chen-Hsun Ho17, Wei-Chieh Huang18, Ying-Ying Chen19
Funding
This work was supported by grants from the National Taiwan University Hospital (Lee BC, 109–004687) and Ministry of Science and Technology of Taiwan (111–2314-B-002–250-MY2).
Author information
Authors and Affiliations
Consortia
Contributions
B-CL project concept and design, data collection, imaging analysis, data analysis, write up. H-HT project concept and design, imaging analysis. Z-WC project concept and design, critical revisions. C-CC project concept and design. L-KT project concept and design. J-ZH data collection, imaging analysis. Y-YC project concept and design. C-HT project concept and design. C-HC critical revisions. C-WL critical revisions. C-TP critical revisions. C-SH critical revisiśons. V-CW project concept and design, data collection, critical revisions. Y-HL project concept and design, data collection, imaging analysis, critical revisions.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, BC., Tsai, HH., Chen, ZW. et al. Aldosteronism is associated with more severe cerebral small vessel disease in hypertensive intracerebral hemorrhage. Hypertens Res 47, 608–617 (2024). https://doi.org/10.1038/s41440-023-01458-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-023-01458-w
Keywords
This article is cited by
-
Prediction of endogenous mineralocorticoid receptor activity by depressor effects of mineralocorticoid receptor antagonists in patients with primary aldosteronism
Hypertension Research (2024)
-
Preface-various factors in the management of blood pressure
Hypertension Research (2024)
-
Possible relationship between primary aldosteronism and small vessel disease
Hypertension Research (2023)