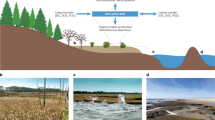
Abstract
Coastal seagrass, mangrove and salt-marsh ecosystems—also termed blue-carbon ecosystems—play an important role in the global carbon cycle. Much of the organic carbon they store rests in soils that have accumulated over thousands of years. Rapidly changing climate and environmental conditions, including sea-level rise, warming, eutrophication and landscape development, will impact decomposition and thus the global reservoir of blue soil organic carbon. Yet, it remains unclear how these disturbances will affect the key biogeochemical mechanisms controlling decomposition—mineral protection, redox zonation, water content and movement, and plant–microbe interactions. We assess the spatial and temporal scales over which decomposition mechanisms operate and how their effectiveness may change following disturbances. We suggest that better integration of decomposition mechanisms into blue-carbon models may improve predictions of soil organic carbon stores and facilitate incorporation of coastal vegetated ecosystems into global budgets and management tools.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
National Academies of Sciences, Engineering, and Medicine Negative Emissions Technologies and Reliable Sequestration: A Research Agenda (The National Academies Press, 2019).
Duarte, C. M., Losada, I. J., Hendriks, I. E., Mazarrasa, I. & Marbà, N. The role of coastal plant communities for climate change mitigation and adaptation. Nat. Clim. Change 3, 961–968 (2013).
Ouyang, X. & Lee, S. Y. Updated estimates of carbon accumulation rates in coastal marsh sediments. Biogeosciences 11, 5057–5071 (2014).
Atwood, T. B. et al. Global patterns in mangrove soil carbon stocks and losses. Nat. Clim. Change 7, 523–528 (2017).
Fourqurean, J. W. et al. Seagrass ecosystems as a globally significant carbon stock. Nat. Geosci. 5, 505–509 (2012).
Hinson, A. L. et al. The spatial distribution of soil organic carbon in tidal wetland soils of the continental United States. Glob. Change Biol. 23, 5468–5480 (2017).
Holmquist, J. R. et al. Accuracy and precision of tidal wetland soil carbon mapping in the conterminous United States. Sci. Rep. 8, 9478 (2018).
Hopkinson, C. S., Morris, J. T., Fagherazzi, S., Wollheim, W. M. & Raymond, P. A. Lateral marsh edge erosion as a source of sediments for vertical marsh accretion. J. Geophys. Res. -Biogeo. 123, 2444–2465 (2018).
Pendleton, L. et al. Estimating global “blue carbon” emissions from conversion and degradation of vegetated coastal ecosystems. PLoS ONE 7, e43542 (2012).
Portnoy, J. W. & Giblin, A. E. Biogeochemical effects of seawater restoration to diked salt marshes. Ecol. Appl. 7, 1054–1063 (1997).
Sanderman, J. et al. A global map of mangrove forest soil carbon at 30 m spatial resolution. Environ. Res. Lett. 13, 055002 (2018).
Ewers Lewis, C. J., Carnell, P. E., Sanderman, J., Baldock, J. A. & Macreadie, P. I. Variability and vulnerability of coastal ‘blue carbon’ stocks: a case study from southeast Australia. Ecosystems 21, 263–279 (2018).
Belshe, E. F., Mateo, M. A., Gillis, L., Zimmer, M. & Teichberg, M. Muddy waters: unintentional consequences of blue carbon research obscure our understanding of organic carbon dynamics in seagrass ecosystems. Front. Mar. Sci. 4, 125 (2017).
Arndt, S. et al. Quantifying the degradation of organic matter in marine sediments: a review and synthesis. Earth-Sci. Rev. 123, 53–86 (2013).
Schmidt, M. W. I. et al. Persistence of soil organic matter as an ecosystem property. Nature 478, 49–56 (2011).
Kelleway, J. J., Saintilan, N., Macreadie, P. I. & Ralph, P. J. Sedimentary factors are key predictors of carbon storage in SE Australian saltmarshes. Ecosystems 19, 865–880 (2016).
Kirwan, M. L. & Mudd, S. M. Response of salt-marsh carbon accumulation to climate change. Nature 489, 550–553 (2012).
Kirwan, M. L., Guntenspergen, G. R. & Langley, J. A. Temperature sensitivity of organic-matter decay in tidal marshes. Biogeosciences 11, 4801–4808 (2014).
Morris, J. T. & Bowden, W. B. A mechanistic, numerical model of sedimentation, mineralization, and decomposition for marsh sediments. Soil Sci. Soc. Am. J. 50, 96–105 (1986).
Fagherazzi, S. et al. Numerical models of salt marsh evolution: ecological, geomorphic, and climatic factors. Rev. Geophys. 50, RG1002 (2012).
Day, J. W. Jr et al. Soil accretionary dynamics, sea-level rise and the survival of wetlands in Venice Lagoon: a field and modelling approach. Estuar. Coast. Shelf Sci. 49, 607–628 (1999).
Lehmann, J. & Kleber, M. The contentious nature of soil organic matter. Nature 528, 60–68 (2015).
Morris, J. T. et al. Contributions of organic and inorganic matter to sediment volume and accretion in tidal wetlands at steady state. Earth’s. Future 4, 110–121 (2016).
Morris, J. T., Sundareshwar, P. V., Nietch, C. T., Kjerfve, B. & Cahoon, D. R. Responses of coastal wetlands to rising sea level. Ecology 83, 2869–2877 (2002).
Yang, J., Gao, J., Liu, B. & Zhang, W. Sediment deposits and organic carbon sequestration along mangrove coasts of the Leizhou Peninsula, southern China. Estuar. Coast. Shelf Sci. 136, 3–10 (2014).
Zhou, J., Wu, Y., Kang, Q. & Zhang, J. Spatial variations of carbon, nitrogen, phosphorous and sulfur in the salt marsh sediments of the Yangtze Estuary in China. Estuar. Coast. Shelf Sci. 71, 47–59 (2007).
Bouillon, S. & Boschker, H. T. S. Bacterial carbon sources in coastal sediments: a cross-system analysis based on stable isotope data of biomarkers. Biogeosciences 3, 175–185 (2006).
Hedges, J. I. & Keil, R. G. Sedimentary organic matter preservation: an assessment and speculative synthesis. Mar. Chem. 49, 81–115 (1995).
Keil, R. G. & Mayer, L. M. in Treatise on Geochemistry 2nd edn (ed. Turekian, K. K.) 337–359 (Elsevier, 2014).
Blair, N. E. & Aller, R. C. The fate of terrestrial organic carbon in the marine environment. Annu. Rev. Mar. Sci. 4, 401–423 (2012).
Zhao, B. et al. The role of reactive iron in the preservation of terrestrial organic carbon in estuarine sediments. J. Geophys. Res. -Biogeo. 123, 3556–3569 (2018).
Liu, Z. & Lee, C. Drying effects on sorption capacity of coastal sediment: the importance of architecture and polarity of organic matter. Geochim. Cosmochim. Acta 70, 3313–3324 (2006).
Liu, Z. & Lee, C. The role of organic matter in the sorption capacity of marine sediments. Mar. Chem. 105, 240–257 (2007).
Canfield, D. E. Factors influencing organic carbon preservation in marine sediments. Chem. Geol. 114, 315–329 (1994).
Cook, P. L. M., Wenzhöfer, F., Glud, R. N., Janssen, F. & Huettel, M. Benthic solute exchange and carbon mineralization in two shallow subtidal sandy sediments: effect of advective pore-water exchange. Limnol. Oceanogr. 52, 1943–1963 (2007).
Cloern, J. E., Canuel, E. A. & Harris, D. Stable carbon and nitrogen isotope composition of aquatic and terrestrial plants of the San Francisco Bay estuarine system. Limnol. Oceanogr. 47, 713–729 (2002).
Trevathan-Tackett, S. M. et al. A global assessment of the chemical recalcitrance of seagrass tissues: implications for long-term carbon sequestration. Front. Plant Sci. 8, 925 (2017).
Kristensen, E., Bouillon, S., Dittmar, T. & Marchand, C. Organic carbon dynamics in mangrove ecosystems: a review. Aquat. Bot. 89, 201–219 (2008).
Enríquez, S., Duarte, C. M. & Sand-Jensen, K. Patterns in decomposition rates among photosynthetic organisms: the importance of detritus C:N:P content. Oecologia 94, 457–471 (1993).
Brodersen, K. E. et al. Oxygen consumption and sulfate reduction in vegetated coastal habitats: effects of physical disturbance. Front. Mar. Sci. 6, 14 (2019).
Ward, L. G., Zaprowski, B. J., Trainer, K. D. & Davis, P. T. Stratigraphy, pollen history and geochronology of tidal marshes in a Gulf of Maine estuarine system: climatic and relative sea level impacts. Mar. Geol. 256, 1–17 (2008).
Spivak, A. C. & Reeve, J. Rapid cycling of recently fixed carbon in a Spartina alterniflora system: a stable isotope tracer experiment. Biogeochemistry 125, 97–114 (2015).
Fontaine, S. et al. Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature 450, 277–280 (2007).
Klotzbücher, T., Kaiser, K., Guggenberger, G., Gatzek, C. & Kalbitz, K. A new conceptual model for the fate of lignin in decomposing plant litter. Ecology 92, 1052–1062 (2011).
Fahimipour, A. K. et al. Global-scale structure of the eelgrass microbiome. Appl. Environ. Microbiol. 83, e03391–16 (2017).
Kearns, P. J. et al. Nutrient enrichment induces dormancy and decreases diversity of active bacteria in salt marsh sediments. Nat. Commun. 7, 12881 (2016).
Balaban, N. Q., Merrin, J., Chait, R., Kowalik, L. & Leibler, S. Bacterial persistence as a phenotypic switch. Science 305, 1622–1625 (2004).
Cúcio, C., Overmars, L., Engelen, A. H. & Muyzer, G. Metagenomic analysis shows the presence of bacteria related to free-living forms of sulfur-oxidizing chemolithoautotrophic symbionts in the rhizosphere of the seagrass Zostera marina. Front. Mar. Sci. 5, 171 (2018).
Bulseco, A. N. et al. Nitrate addition stimulates microbial decomposition of organic matter in salt marsh sediments. Glob. Change Biol. https://doi.org/10.1111/gcb.14726 (2019).
Liang, C., Schimel, J. P. & Jastrow, J. D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2, 17105 (2017).
Kirwan, M. L. & Gedan, K. B. Sea-level driven land conversion and the formation of ghost forests. Nat. Clim. Change 9, 450–457 (2019).
Saunders, M. I. et al. Coastal retreat and improved water quality mitigate losses of seagrass from sea level rise. Glob. Change Biol. 19, 2569–2583 (2013).
Fagherazzi, S. et al. Sea level rise and the dynamics of the marsh-upland boundary. Front. Environ. Sci. 7, 25 (2019).
Schuerch, M. et al. Future response of global coastal wetlands to sea-level rise. Nature 561, 231–234 (2018).
Mueller, P., Jensen, K. & Megonigal, J. P. Plants mediate soil organic matter decomposition in response to sea level rise. Glob. Change Biol. 22, 404–414 (2016).
Freeman, C., Ostle, N. J., Fenner, N. & Kang, H. A regulatory role for phenol oxidase during decomposition in peatlands. Soil Biol. Biochem. 36, 1663–1667 (2004).
Craft, C. et al. Forecasting the effects of accelerated sea-level rise on tidal marsh ecosystem services. Front. Ecol. Environ. 7, 73–78 (2009).
Weston, N. B., Dixon, R. E. & Joye, S. B. Ramifications of increased salinity in tidal freshwater sediments: geochemistry and microbial pathways of organic matter mineralization. J. Geophys. Res. -Biogeo. 111, G01009 (2006).
Davidson, E. A. & Janssens, I. A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440, 165–173 (2006).
Melillo, J. M. et al. Long-term pattern and magnitude of soil carbon feedback to the climate system in a warming world. Science 358, 101–105 (2017).
Pold, G., Grandy, A. S., Melillo, J. M. & DeAngelis, K. M. Changes in substrate availability drive carbon cycle response to chronic warming. Soil Biol. Biochem. 110, 68–78 (2017).
von Lützow, M. & Kögel-Knabner, I. Temperature sensitivity of soil organic matter decomposition—what do we know? Biol. Fert. Soils 46, 1–15 (2009).
Wilson, R. M. et al. Stability of peatland carbon to rising temperatures. Nat. Commun. 7, 13723 (2016).
Osland, M. J., Enwright, N., Day, R. H. & Doyle, T. W. Winter climate change and coastal wetland foundation species: salt marshes vs. mangrove forests in the southeastern United States. Glob. Change Biol. 19, 1482–1494 (2013).
Root, T. L. et al. Fingerprints of global warming on wild animals and plants. Nature 421, 57–60 (2003).
Saintilan, N., Wilson, N. C., Rogers, K., Rajkaran, A. & Krauss, K. W. Mangrove expansion and salt marsh decline at mangrove poleward limits. Glob. Change Biol. 20, 147–157 (2014).
Mafi-Gholami, D., Zenner, E. K., Jaafari, A. & Ward, R. D. Modeling multi-decadal mangrove leaf area index in response to drought along the semi-arid southern coasts of Iran. Sci. Total Environ. 656, 1326–1336 (2019).
McKee, K. L., Mendelssohn, I. A. & Materne, M. D. Acute salt marsh dieback in the Mississippi River deltaic plain: a drought-induced phenomenon? Glob. Ecol. Biogeogr. 13, 65–73 (2004).
Jordà, G., Marbà, N. & Duarte, C. M. Mediterranean seagrass vulnerable to regional climate warming. Nat. Clim. Change 2, 821–824 (2012).
Van der Putten, W. H., Macel, M. & Visser, M. E. Predicting species distribution and abundance responses to climate change: why it is essential to include biotic interactions across trophic levels. Philos. Trans. R. Soc. B 365, 2025–2034 (2010).
Marbà, N. et al. Impact of seagrass loss and subsequent revegetation on carbon sequestration and stocks. J. Ecol. 103, 296–302 (2015).
Sanford, E., Holzman, S. B., Haney, R. A., Rand, D. M. & Bertness, M. D. Larval tolerance, gene flow, and the northern geographic range limit of fiddler crabs. Ecology 87, 2882–2894 (2006).
Kostka, J. E. et al. The rates and pathways of carbon oxidation in bioturbated saltmarsh sediments. Limnol. Oceanogr. 47, 230–240 (2002).
Kristensen, E. & Alongi, D. M. Control by fiddler crabs (Uca vocans) and plant roots (Avicennia marina) on carbon, iron, and sulfur biogeochemistry in mangrove sediment. Limnol. Oceanogr. 51, 1557–1571 (2006).
Burkholder, J. M., Tomasko, D. A. & Touchette, B. W. Seagrasses and eutrophication. J. Exp. Mar. Biol. Ecol. 350, 46–72 (2007).
Turner, R. E. et al. Salt marshes and eutrophication: an unsustainable outcome. Limnol. Oceanogr. 54, 1634–1642 (2009).
Sanders, C. J. et al. Elevated rates of organic carbon, nitrogen, and phosphorus accumulation in a highly impacted mangrove wetland. Geophys. Res. Lett. 41, 2475–2480 (2014).
Bowen, J. L., Crump, B. C., Deegan, L. A. & Hobbie, J. E. Increased supply of ambient nitrogen has minimal effect on salt marsh bacterial production. Limnol. Oceanogr. 54, 713–722 (2009).
Nowinski, N. S., Trumbore, S. E., Schuur, E. A. G., Mack, M. C. & Shaver, G. R. Nutrient addition prompts rapid destabilization of organic matter in an Arctic tundra ecosystem. Ecosystems 11, 16–25 (2008).
Richards, D. R. & Friess, D. A. Rates and drivers of mangrove deforestation in Southeast Asia, 2000–2012. Proc. Natl Acad. Sci. USA 113, 344–349 (2016).
Osland, M. J. et al. Ecosystem development after mangrove wetland creation: plant–soil change across a 20-year chronosequence. Ecosystems 15, 848–866 (2012).
Greening, H. & Janicki, A. Toward reversal of eutrophic conditions in a subtropical estuary: water quality and seagrass response to nitrogen loading reductions in Tampa Bay, Florida, USA. Environ. Manag. 38, 163–178 (2006).
Dahl, M. et al. Sediment properties as important predictors of carbon storage in Zostera marina meadows: a comparison of four European areas. PLoS ONE 11, e0167493 (2016).
Röhr, M. E., Boström, C., Canal-Vergés, P. & Holmer, M. Blue carbon stocks in Baltic Sea eelgrass (Zostera marina) meadows. Biogeosciences 13, 6139–6153 (2016).
Serrano, O. et al. Can mud (silt and clay) concentration be used to predict soil organic carbon content within seagrass ecosystems? Biogeosciences 13, 4915–4926 (2016).
Liu, Z., Breecker, D., Mayer, L. M. & Zhong, J. Composition of size-fractioned sedimentary organic matter in coastal environments is affected by difference in physical forcing strength. Org. Geochem. 60, 20–32 (2013).
Arzayus, K. M. & Canuel, E. A. Organic matter degradation in sediments of the York River estuary: effects of biological vs. physical mixing. Geochim. Cosmochim. Acta 69, 455–464 (2005).
Luther, G. W., Ferdelman, T. G., Kostka, J. E., Tsamakis, E. J. & Church, T. M. Temporal and spatial variability of reduced sulfur species (FeS2, S2O3 2−) and porewater parameters in salt marsh sediments. Biogeochemistry 14, 57–88 (1991).
Koch, E. W. Beyond light: physical, geological, and geochemical parameters as possible submersed aquatic vegetation habitat requirements. Estuaries 24, 1–17 (2001).
Bowen, J. L. et al. Lineage overwhelms environmental conditions in determining rhizosphere bacterial community structure in a cosmopolitan invasive plant. Nat. Commun. 8, 433 (2017).
Costanza, R. et al. Changes in the global value of ecosystem services. Glob. Environ. Change 26, 152–158 (2014).
Wong, P. P. et al. in Climate Change 2014: Impacts, Adaptation, and Vulnerability (eds Field, C. B. et al.) 361–409 (Cambridge Univ. Press, 2014).
Hug, L. A. et al. A new view of the tree of life. Nat. Microbiol. 1, 16048 (2016).
Yvon-Durocher, G., Jones, J. I., Trimmer, M., Woodward, G. & Montoya, J. M. Warming alters the metabolic balance of ecosystems. Philos. Trans. R. Soc. B 365, 2117–2126 (2010).
Waycott, M. et al. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc. Natl Acad. Sci. USA 106, 12377–12381 (2009).
Perillo, G., Wolanski, E., Cahoon, D. & Hopkinson, C. S. Coastal Wetlands: An Integrated Ecosystem Approach 2nd edn (Elsevier, 2019).
Hamilton, S. E. & Casey, D. Creation of a high spatio-temporal resolution global database of continuous mangrove forest cover for the 21st century (CGMFC-21). Glob. Ecol. Biogeogr. 25, 729–738 (2016).
Todd-Brown, K. E. O. et al. Causes of variation in soil carbon simulations from CMIP5 Earth system models and comparison with observations. Biogeosciences 10, 1717–1736 (2013).
Wang, Y. P. et al. Responses of two nonlinear microbial models to warming and increased carbon input. Biogeosciences 13, 887–902 (2016).
Acknowledgements
A.C.S. was supported by Woods Hole Sea Grant (NA14OAR4170104) and NOAA NSC (NA14NOS4190145); J.L.B. by a CAREER award from NSF DEB (1350491/1719446); E.A.C. by NSF-DEB 1556554; and C.S.H. by NSF OCE-1238212, NSF-OCE 1637630, NSF-OCE 12-37140 and NSF-OCE18-32178.
Author information
Authors and Affiliations
Contributions
A.C.S. conceived and wrote initial drafts. All authors contributed to idea development and manuscript writing and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Spivak, A.C., Sanderman, J., Bowen, J.L. et al. Global-change controls on soil-carbon accumulation and loss in coastal vegetated ecosystems. Nat. Geosci. 12, 685–692 (2019). https://doi.org/10.1038/s41561-019-0435-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41561-019-0435-2
This article is cited by
-
The inclusion of Amazon mangroves in Brazil’s REDD+ program
Nature Communications (2024)
-
Impact of COVID-19 lockdown on methane related activities in a tropical estuarine mangrove ecosystem
Journal of Coastal Conservation (2024)
-
Organic matter decomposition and associated microbial communities in wetlands: insights from tropical and subtropical Melaleuca forests in Australia
Hydrobiologia (2024)
-
Climate-driven tradeoffs between landscape connectivity and the maintenance of the coastal carbon sink
Nature Communications (2023)
-
Attaining freshwater and estuarine-water soil saturation in an ecosystem-scale coastal flooding experiment
Environmental Monitoring and Assessment (2023)