Abstract
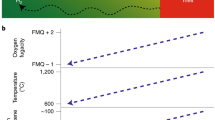
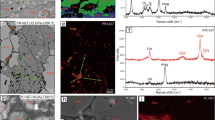
Subduction zones are key regions of chemical and mass transfer between the Earth’s surface and mantle. During subduction, oxidized material is carried into the mantle and large amounts of water are released due to the breakdown of hydrous minerals such as lawsonite. Dehydration accompanied by the release of oxidizing species may play a key role in controlling redox changes in the subducting slab and overlying mantle wedge. Here we present measurements of oxygen fugacity, using garnet–epidote oxybarometry, together with analyses of the stable iron isotope composition of zoned garnets from Sifnos, Greece. We find that the garnet interiors grew under relatively oxidized conditions whereas garnet rims record more reduced conditions. Garnet δ56Fe increases from core to rim as the system becomes more reduced. Thermodynamic analysis shows that this change from relatively oxidized to more reduced conditions occurred during lawsonite dehydration. We conclude that the garnets maintain a record of progressive dehydration and that the residual mineral assemblages within the slab became more reduced during progressive subduction-zone dehydration. This is consistent with the hypothesis that lawsonite dehydration accompanied by the release of oxidizing species, such as sulfate, plays an important and measurable role in the global redox budget and contributes to sub-arc mantle oxidation in subduction zones.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data generated or analysed during this study are included in this published article and its Supplementary Information files.
References
Frost, B. R. in Oxide Minerals: Petrologic and Magnetic Significance. Reviews in Mineralogy Vol. 25 (ed. Lindsley, D. H.) 1–9 (Mineralogical Society of America, 1991).
Schmidt, M. W. & Poli, S. Experimentally based water budgets for dehydrating slabs and consequences for arc magma generation. Earth Planet. Sci. Lett. 163, 361–379 (1998).
Magni, V., Bouilhol, P. & van Hunen, J. Deep water recycling through time. Geochem. Geophys. Geosyst. 15, 4203–4216 (2014).
Poli, S. & Schmidt, M. W. H2O transport and release in subduction zones: experimental constraints on basaltic and andesitic systems. J. Geophys. Res. Solid Earth 100, 22299–22314 (1995).
Tatsumi, Y. Formation of the volcanic front in subduction zones. Geophys. Res. Lett. 13, 717–720 (1986).
Marschall, H. R. & Schumacher, J. C. Arc magmas sourced from mélange diapirs in subduction zones. Nat. Geosci. 5, 862–867 (2012).
Breeding, C. M., Ague, J. J. & Bröcker, M. Fluid-metasedimentary rock interactions in subduction-zone mélange: implications for the chemical composition of arc magmas. Geology 32, 1041–1044 (2004).
Kelley, K. A. & Cottrell, E. Water and the oxidation state of subduction zone magmas. Science 325, 605–607 (2009).
Parkinson, I. J. & Arculus, R. J. The redox state of subduction zones: insights from arc-peridotites. Chem. Geol. 160, 409–423 (1999).
Evans, K. A., Elburg, M. A. & Kamenetsky, V. S. Oxidation state of subarc mantle. Geology 40, 783–786 (2012).
Debret, B. & Sverjensky, D. A. Highly oxidising fluids generated during serpentinite breakdown in subduction zones. Sci. Rep. 7, 10351 (2017).
Lee, C. T. A. et al. The redox state of arc mantle using Zn/Fe systematics. Nature 468, 681–685 (2010).
Pons, M.-L., Debret, B., Bouilhol, P., Delacour, A. & Williams, H. Zinc isotope evidence for sulfate-rich fluid transfer across subduction zones. Nat. Commun. 7, 13794 (2016).
Tomkins, A. G. & Evans, K. A. Separate zones of sulfate and sulfide release from subducted mafic oceanic crust. Earth Planet. Sci. Lett. 428, 73–83 (2015).
Evans, K. A. The redox budget of subduction zones. Earth-Sci. Rev. 113, 11–32 (2012).
Debret, B. et al. Isotopic evidence for iron mobility during subduction. Geology 44, 215–218 (2016).
Walters, J. B., Cruz-Uribe, A. M. & Marschall, H. R. Isotopic compositions of sulfides in exhumed high‐pressure terranes: implications for sulfur cycling in subduction zones. Geochem. Geophys. Geosyst. 20, 3347–3374 (2019).
Bénard, A. et al. Oxidising agents in sub-arc mantle melts link slab devolatilisation and arc magmas. Nat. Commun. 9, 3500 (2018).
Canil, D. & Fellows, S. A. Sulphide–sulphate stability and melting in subducted sediment and its role in arc mantle redox and chalcophile cycling in space and time. Earth Planet. Sci. Lett. 470, 73–86 (2017).
Ague, J. J., Baxter, E. F. & Eckert, J. O.Jr. High fO2 during sillimanite zone metamorphism of part of the Barrovian type locality, Glen Clova, Scotland. J. Petrol. 42, 1301–1320 (2001).
Baxter, E. F. & Caddick, M. J. Garnet growth as a proxy for progressive subduction zone dehydration. Geology 41, 643–646 (2013).
Dragovic, B., Baxter, E. F. & Caddick, M. J. Pulsed dehydration and garnet growth during subduction revealed by zoned garnet geochronology and thermodynamic modeling, Sifnos, Greece. Earth Planet. Sci. Lett. 413, 111–122 (2015).
Dragovic, B., Samanta, L. M., Baxter, E. F. & Selverstone, J. Using garnet to constrain the duration and rate of water-releasing metamorphic reactions during subduction: an example from Sifnos, Greece. Chem. Geol. 314–317, 9–22 (2012).
Polyakov, V. B. & Mineev, S. D. The use of Mossbauer spectroscopy in stable isotope geochemistry. Geochim. Cosmochim. Acta 64, 849–865 (2000).
Schauble, E. A., Rossman, G. R. & Taylor, H. P. Theoretical estimates of equilibrium Fe isotope fractionations from vibrational spectroscopy. Geochim. Cosmochim. Acta 65, 2487–2497 (2001).
Inglis, E. C. et al. The behavior of iron and zinc stable isotopes accompanying the subduction of mafic oceanic crust: a case study from Western Alpine ophiolites. Geochem. Geophys. Geosyst. 18, 2562–2579 (2017).
Donohue, C. L. & Essene, E. J. An oxygen barometer with the assemblage garnet–epidote. Earth Planet. Sci. Lett. 181, 459–472 (2000).
Boundy, T. M., Donohue, C. L., Essene, E. J., Mezger, K. & Austrheim, H. Discovery of eclogite facies carbonate rocks from the Lindås Nappe, Caledonides, Western Norway. J. Metamorph. Geol. 20, 649–667 (2002).
Cao, Y., Song, S. G., Niu, Y. L., Jung, H. & Jin, Z. M. Variation of mineral composition, fabric and oxygen fugacity from massive to foliated eclogites during exhumation of subducted ocean crust in the North Qilian suture zone, NW China. J. Metamorph. Geol. 29, 699–720 (2011).
Mattinson, C. G., Zhang, R. Y., Tsujimori, T. & Liou, J. G. Epidote-rich talc-kyanite-phengite eclogites, Sulu terrane, eastern China: P-T-fo2 estimates and the significance of the epidote-talc assemblage in eclogite. Am. Mineral. 89, 1772–1783 (2004).
Groppo, C., Forster, M., Lister, G. & Compagnoni, R. Glaucophane schists and associated rocksfrom Sifnos (Cyclades, Greece): new constraints on the P-T evolution from oxidized systems. Lithos 109, 254–273 (2009).
Hill, P. S., Schauble, E. A. & Young, E. D. Effects of changing solution chemistry on Fe3+/Fe2+ isotope fractionation in aqueous Fe–Cl solutions. Geochim. Cosmochim. Acta 74, 6669–6689 (2010).
Holland, T. J. B. & Powell, R. An internally consistent thermodynamic data set for phases of petrological interest. J. Metamorph. Geol. 16, 309–343 (1998).
Powell, R. & Holland, T. J. B. An internally consistent dataset with uncertainties and correlations: 3. Applications to geobarometry, worked examples and a computer program. J. Metamorph. Geol. 6, 173–204 (1988).
Powell, R. & Holland, T. Optimal geothermometry and geobarometry. Am. Mineral. 79, 120–133 (1994).
Quinn, R. J., Valley, J. W., Page, F. Z. & Fournelle, J. H. Accurate determination of ferric iron in garnets. Am. Mineral. 101, 1704–1707 (2016).
Barr, H. Preliminary fluid inclusion studies in a high-grade blueschist terrain, Syros, Greece. Mineral. Mag. 54, 159–168 (1990).
Palin, R. M., Weller, O. M., Waters, D. J. & Dyck, B. Quantifying geological uncertainty in metamorphic phase equilibria modelling; a Monte Carlo assessment and implications for tectonic interpretations. Geosci. Front. 7, 591–607 (2016).
Pollington, A. D. & Baxter, E. F. High precision microsampling and preparation of zoned garnet porphyroblasts for Sm–Nd geochronology. Chem. Geol. 281, 270–282 (2011).
Dauphas, N. et al. Chromatographic separation and multicollection-ICPMS analysis of iron. Investigating mass-dependent and-independent isotope effects. Anal. Chem. 76, 5855–5863 (2004).
Weyer, S. & Schwieters, J. B. High precision Fe isotope measurements with high mass resolution MC-ICPMS. Int. J. Mass Spectrom. 226, 355–368 (2003).
Millet, M. A., Baker, J. A. & Payne, C. E. Ultra-precise stable Fe isotope measurements by high resolution multiple-collector inductively coupled plasma mass spectrometry with a 57Fe–58Fe double spike. Chem. Geol. 304, 18–25 (2012).
Hibbert, K. E. J., Williams, H. M., Kerr, A. C. & Puchtel, I. S. Iron isotopes in ancient and modern komatiites: evidence in support of an oxidised mantle from Archean to present. Earth Planet. Sci. Lett. 321, 198–207 (2012).
Sossi, P. A., Halverson, G. P., Nebel, O. & Eggins, S. M. Combined separation of Cu, Fe and Zn from rock matrices and improved analytical protocols for stable isotope determination. Geostand. Geoanal. Res. 39, 129–149 (2015).
Connolly, J. A. D. The geodynamic equation of state: what and how. Geochem. Geophys. Geosyst. 10, 10 (2009).
Diener, J. F. A. & Powell, R. Revised activity–composition models for clinopyroxene and amphibole. J. Metamorph. Geol. 30, 131–142 (2012).
White, R. W., Powell, R. & Holland, T. J. B. Progress relating to calculation of partial melting equilibria for metapelites. J. Metamorph. Geol. 25, 511–527 (2007).
Auzanneau, E., Schmidt, M. W., Vielzeuf, D. & Connolly, J. D. Titanium in phengite: a geobarometer for high temperature eclogites. Contrib. Mineral. Petrol. 159, 1 (2010).
Coggan, R. & Holland, T. J. B. Mixing properties of phengitic micas and revised garnet–phengite thermometers. J. Metamorph. Geol. 20, 683–696 (2002).
Powell, R., Holland, T. J. B. H. & Worley, B. Calculating phase diagrams involving solid solutions via non‐linear equations, with examples using THERMOCALC. J. Metamorph. Geol. 16, 577–588 (1998).
Fuhrman, M. L. & Lindsley, D. H. Ternary-feldspar modeling and thermometry. Am. Mineral. 73, 201–215 (1988).
White, R. W., Powell, R. & Clarke, G. L. The interpretation of reaction textures in Fe-rich metapelitic granulites of the Musgrave Block, central Australia: constraints from mineral equilibria calculations in the system K2O–FeO–MgO–Al2O3–SiO2–H2O–TiO2–Fe2O3. J. Metamorph. Geol. 20, 41–55 (2002).
White, R. W., Powell, R., Holland, T. J. B. & Worley, B. A. The effect of TiO2 and Fe2O3 on metapelitic assemblages at greenschist and amphibolite facies conditions: mineral equilibria calculations in the system K2O–FeO–MgO–Al2O3–SiO2–H2O–TiO2–Fe2O3. J. Metamorph. Geol. 18, 497–512 (2002).
Holland, T. & Powell, R. Activity–composition relations for phases in petrological calculations: an asymmetric multicomponent formulation. Contrib. Mineral. Petrol. 145, 492–501 (2003).
Marmo, B. A., Clarke, G. L. & Powell, R. Fractionation of bulk rock composition due to porphyroblast growth: effects on eclogite facies mineral equilibria, Pam Peninsula, New Caledonia. J. Metamorph. Geol. 20, 151–165 (2002).
Brooks, H. L., Dragovic, B., Lamadrid, H. M., Caddick, M. J. & Bodnar, R. Fluid capture during exhumation of subducted lithologies: a fluid inclusion study from Sifnos, Greece. Lithos 332-333, 120–134 (2019).
Acknowledgements
E.F.B. acknowledges support from NSF grants EAR-0547999 for sample collection and OIA-1545903 for support during this project. A.R.G., B.D. and P.G.S. also acknowledge support from OIA-1545903. E.C.I. was supported as a postdoctoral research assistant on ERC starting grant PRISTINE: 637503 awarded to F. Moynier (IPG Paris).
Author information
Authors and Affiliations
Contributions
A.R.G. was responsible for the oxybarometry calculations, clean-lab preparation and analysis of Fe isotope compositions and wrote the manuscript. E.C.I. performed Fe isotope compositional analysis and contributed to method development. B.D. was responsible for P–T modelling. P.G.S. performed clean-lab preparation, oxybarometry calculations and method development. E.F.B. and K.W.B. contributed by designing the project. All authors contributed to analysis and interpretation of data and editing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Primary Handling Editor(s): Melissa Plail; Heike Langenberg.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary figures and tables.
Supplementary Data
Supplementary Tables 1–7.
Rights and permissions
About this article
Cite this article
Gerrits, A.R., Inglis, E.C., Dragovic, B. et al. Release of oxidizing fluids in subduction zones recorded by iron isotope zonation in garnet. Nat. Geosci. 12, 1029–1033 (2019). https://doi.org/10.1038/s41561-019-0471-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41561-019-0471-y
This article is cited by
-
Slab-derived devolatilization fluids oxidized by subducted metasedimentary rocks
Nature Geoscience (2022)
-
Redox processes in subduction zones: Progress and prospect
Science China Earth Sciences (2020)
-
Crustal reworking and hydration: insights from element zoning and oxygen isotopes of garnet in high-pressure rocks (Sesia Zone, Western Alps)
Contributions to Mineralogy and Petrology (2020)