Abstract
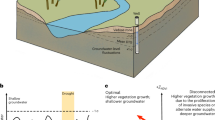
Geogenic groundwater arsenic (As) contamination is pervasive in many aquifers in south and southeast Asia. It is feared that recent increases in groundwater abstractions could induce the migration of high-As groundwaters into previously As-safe aquifers. Here we study an As-contaminated aquifer in Van Phuc, Vietnam, located ~10 km southeast of Hanoi on the banks of the Red River, which is affected by large-scale groundwater abstraction. We used numerical model simulations to integrate the groundwater flow and biogeochemical reaction processes at the aquifer scale, constrained by detailed hydraulic, environmental tracer, hydrochemical and mineralogical data. Our simulations provide a mechanistic reconstruction of the anthropogenically induced spatiotemporal variations in groundwater flow and biogeochemical dynamics and determine the evolution of the migration rate and mass balance of As over several decades. We found that the riverbed–aquifer interface constitutes a biogeochemical reaction hotspot that acts as the main source of elevated As concentrations. We show that a sustained As release relies on regular replenishment of river muds rich in labile organic matter and reactive iron oxides and that pumping-induced groundwater flow may facilitate As migration over distances of several kilometres into adjacent aquifers.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The geochemical data analysed during this study are included in this article in the supplementary information in Supplementary Tables 1 and Supplementary Tables 2. The groundwater age data analysed during this study has been published and is available in van Geen et al.7 and Stahl et al.10 (Supplementary Table 1). The solid phase chemistry data at the site was available from Eiche et al.32 and Eiche21.
Code availability
All codes used as part of this study are publicly available and can be accessed freely. The USGS flow model MODFLOW37 (https://www.usgs.gov/software/software-modflow) was used to perform the groundwater flow simulations, whereas the reactive multi-component transport model PHT3D38 was used to simulate solute and reactive transport processes (http://www.pht3d.org/). PHT3D couples the 3D transport simulator MT3DMS39 with the USGS geochemical model PHREEQC-240. The PEST++ software suite41 was employed for model calibration and uncertainty analysis (http://www.pesthomepage.org/).
References
Fendorf, S., Michael, H. A. & van Geen, A. Spatial and temporal variations of groundwater arsenic in South and Southeast Asia. Science 328, 1123–1127 (2010).
Winkel, L. H. et al. Arsenic pollution of groundwater in Vietnam exacerbated by deep aquifer exploitation for more than a century. Proc. Natl Acad. Sci. 108, 1246–1251 (2011).
Postma, D. et al. Groundwater arsenic concentrations in Vietnam controlled by sediment age. Nat. Geosci. 5, 656–661 (2012).
Berg, M. et al. Hydrological and sedimentary controls leading to arsenic contamination of groundwater in the Hanoi area, Vietnam: the impact of iron–arsenic ratios, peat, river bank deposits, and excessive groundwater abstraction. Chem. Geol. 249, 91–112 (2008).
Radloff, K. et al. Reversible adsorption and flushing of arsenic in a shallow, Holocene aquifer of Bangladesh. Appl. Geochem. 77, 142–157 (2017).
Neumann, R. B. et al. Anthropogenic influences on groundwater arsenic concentrations in Bangladesh. Nat. Geosci. 3, 46–52 (2010).
van Geen, A. et al. Retardation of arsenic transport through a Pleistocene aquifer. Nature 501, 204–207 (2013).
Khan, M. R. et al. Megacity pumping and preferential flow threaten groundwater quality. Nat. Commun. 7, 12833 (2016).
Michael, H. A. & Khan, M. R. Impacts of physical and chemical aquifer heterogeneity on basin-scale solute transport: vulnerability of deep groundwater to arsenic contamination in Bangladesh. Adv. Water Resour. 98, 147–158 (2016).
Stahl, M. O. et al. River bank geomorphology controls groundwater arsenic concentrations in aquifers adjacent to the Red River, Hanoi Vietnam. Water Resour. Res. 52, 6321–6334 (2016).
McArthur, J., Ravenscroft, P., Safiulla, S. & Thirlwall, M. Arsenic in groundwater: testing pollution mechanisms for sedimentary aquifers in Bangladesh. Water Resour. Res. 37, 109–117 (2001).
McArthur, J. et al. Natural organic matter in sedimentary basins and its relation to arsenic in anoxic ground water: the example of West Bengal and its worldwide implications. Appl. Geochem. 19, 1255–1293 (2004).
Meharg, A. A. et al. Codeposition of organic carbon and arsenic in Bengal Delta aquifers. Environ. Sci. Technol. 40, 4928–4935 (2006).
Postma, D. et al. Arsenic in groundwater of the Red River floodplain, Vietnam: controlling geochemical processes and reactive transport modeling. Geochim. Cosmochim. Acta 71, 5054–5071 (2007).
Polizzotto, M. L., Kocar, B. D., Benner, S. G., Sampson, M. & Fendorf, S. Near-surface wetland sediments as a source of arsenic release to ground water in Asia. Nature 454, 505–509 (2008).
Stuckey, J. W., Schaefer, M. V., Kocar, B. D., Benner, S. G. & Fendorf, S. Arsenic release metabolically limited to permanently water-saturated soil in Mekong Delta. Nat. Geosci. 9, 70–76 (2016).
Berg, M. et al. Arsenic contamination of groundwater and drinking water in Vietnam: a human health threat. Environ. Sci. Technol. 35, 2621–2626 (2001).
Harvey, C. F. et al. Arsenic mobility and groundwater extraction in Bangladesh. Science 298, 1602–1606 (2002).
Horneman, A. et al. Decoupling of As and Fe release to Bangladesh groundwater under reducing conditions. Part 1: Evidence from sediment profiles. Geochim. Cosmochim. Acta 68, 3459–3473 (2004).
Islam, F. S. et al. Role of metal-reducing bacteria in arsenic release from Bengal delta sediments. Nature 430, 68–71 (2004).
Eiche, E. Arsenic Mobilization Processes in the Red River Delta, Vietnam: Towards a Better Understanding of the Patchy Distribution of Dissolved Arsenic in Alluvial Deposits (Karlsruher Mineralogische und Geochemische Hefte 37, KIT Scientific, 2009).
Frei, F. Groundwater Dynamics and Arsenic Mobilization near Hanoi (Vietnam) Assessed Using Noble Gases and Tritium Diploma Thesis, ETH Swiss Federal Institute of Technology, Department of Environmental Sciences (2007).
Eiche, E. et al. Origin and availability of organic matter leading to arsenic mobilisation in aquifers of the Red River Delta, Vietnam. Appl. Geochem. 77, 184–193 (2017).
Postma, D. et al. Mobilization of arsenic and iron from Red River floodplain sediments, Vietnam. Geochim. Cosmochim. Acta 74, 3367–3381 (2010).
Postma, D. et al. Fate of arsenic during Red River water infiltration into aquifers beneath Hanoi, Vietnam. Environ. Sci. Technol. 51, 838–845 (2017).
Larsen, F. et al. Controlling geological and hydrogeological processes in an arsenic contaminated aquifer on the Red River flood plain, Vietnam. Appl. Geochem. 23, 3099–3115 (2008).
McClain, M. E. et al. Biogeochemical hot spots and hot moments at the interface of terrestrial and aquatic ecosystems. Ecosystems 6, 301–312 (2003).
Cheng, F. Y. & Basu, N. B. Biogeochemical hotspots: role of small water bodies in landscape nutrient processing. Water Resour. Res. 53, 5038–5056 (2017).
Hedin, L. O. et al. Thermodynamic constraints on nitrogen transformations and other biogeochemical processes at soil–stream interfaces. Ecology 79, 684–703 (1998).
Kocar, B. D. & Fendorf, S. in Interdisciplinary Studies on Environmental Chemistry—Environmental Pollution and Ecotoxicology (eds Kawaguchi, M. et al.) 117–124 (TERRAPUB, 2012).
Rathi, B., Neidhardt, H., Berg, M., Siade, A. & Prommer, H. Processes governing arsenic retardation on Pleistocene sediments: adsorption experiments and model‐based analysis. Water Resour. Res. 53, 4344–4360 (2017).
Eiche, E. et al. Geochemical processes underlying a sharp contrast in groundwater arsenic concentrations in a village on the Red River delta, Vietnam. Appl. Geochem. 23, 3143–3154 (2008).
van Geen, A. et al. Comparison of arsenic concentrations in simultaneously-collected groundwater and aquifer particles from Bangladesh, India, Vietnam, and Nepal. Appl. Geochem. 23, 3244–3251 (2008).
Neidhardt, H. et al. Insights into arsenic retention dynamics of Pleistocene aquifer sediments by in situ sorption experiments. Water Res. 129, 123–132 (2018).
van Geen, A. et al. Spatial variability of arsenic in 6000 tube wells in a 250 km2 area of Bangladesh. Water Resour. Res. 39, 1140 (2003).
McArthur, J. et al. How paleosols influence groundwater flow and arsenic pollution: a model from the Bengal Basin and its worldwide implication. Water Resour. Res. 44, W11411 (2008).
Harbaugh, A. W. MODFLOW-2005, the US Geological Survey Modular Ground-water Model: The Ground-water Flow Process (US Department of the Interior, US Geological Survey, 2005).
Prommer, H., Barry, D. A. & Zheng, C. MODFLOW/MT3DMS-based reactive multicomponent transport modeling. Ground Water 41, 247–257 (2003).
Zheng, C. & Wang, P. P. MT3DMS: A Modular Three-dimensional Multispecies Transport Model for Simulation of Advection, Dispersion, and Chemical Reactions of Contaminants in Groundwater Systems; Documentation and User’s Guide (U.S. Army Corps of Engineers Document, 1999).
Parkhurst, D. L. & Appelo, C. User’s Guide to PHREEQC (Version 2): A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations Report No. 99-4259 (USGS, 1999).
Welter, D. E., White, J. T., Hunt, R. J. & Doherty, J. E. Approaches in Highly Parameterized Inversion—PEST++ Version 3, a Parameter ESTimation and Uncertainty Analysis Software Suite Optimized for Large Environmental Models Report No. 2328-7055 (USGS, 2015).
Postma, D. & Jakobsen, R. Redox zonation: equilibrium constraints on the Fe(iii)/SO4-reduction interface. Geochim. Cosmochim. Acta 60, 3169–3175 (1996).
Prommer, H., Tuxen, N. & Bjerg, P. L. Fringe-controlled natural attenuation of phenoxy acids in a landfill plume: integration of field-scale processes by reactive transport modeling. Environ. Sci. Technol. 40, 4732–4738 (2006).
Sharma, L., Greskowiak, J., Ray, C., Eckert, P. & Prommer, H. Elucidating temperature effects on seasonal variations of biogeochemical turnover rates during riverbank filtration. J. Hydrol. 428, 104–115 (2012).
Rawson, J. et al. Quantifying reactive transport processes governing arsenic mobility after injection of reactive organic carbon into a Bengal Delta aquifer. Environ. Sci. Technol. 51, 8471–8480 (2017).
Schwertmann, U. Solubility and dissolution of iron oxides. Plant Soil 130, 1–25 (1991).
Appelo, C. A. J., Van der Weiden, M. J. J., Tournassat, C. & Charlet, L. Surface complexation of ferrous iron and carbonate on ferrihydrite and the mobilization of arsenic. Environ. Sci. Technol. 36, 3096–3103 (2002).
Dzombak, D. A. & Morel, F. M. Surface Complexation Modeling: Hydrous Ferric Oxide (John Wiley & Sons, 1990).
Swedlund, P. J. & Webster, J. G. Adsorption and polymerisation of silicic acid on ferrihydrite, and its effect on arsenic adsorption. Water Res. 33, 3413–3422 (1999).
Acknowledgements
This study was supported by the Swiss National Science Foundation (SNSF grant no. IZK0Z2_150435/ IZK0Z2_150435/1 and SNSF grant no. 167821) and the German Research Foundation (DFG grant no. 320059499). M. O. Stahl (Union College), B. Bostick and A. van Geen (Columbia University) contributed to this work through helpful discussions on previous work at the field site. P. Ortega prepared Fig. 1.
Author information
Authors and Affiliations
Contributions
R.K., M.B., I.W. and H.P. conceived the study. M.B. and R.K. provided hydrochemical and tracer data and contributed to the groundwater age, hydraulic and hydrogeochemical interpretation. I.W. and H.P. carried out the flow and reactive transport modelling and J.S., M.B., R.K, I.W. and H.P. contributed to the development of the geochemical conceptual model underpinning the numerical model. A.J.S. undertook flow and solute transport model calibration and contributed to model uncertainty analysis. All authors contributed to writing and editing the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Primary Handling Editors: Tamara Goldin; Melissa Plail.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Supplementary information
Supplementary Information
Supplementary Tables 1–6 and Figs. 1–12.
Rights and permissions
About this article
Cite this article
Wallis, I., Prommer, H., Berg, M. et al. The river–groundwater interface as a hotspot for arsenic release. Nat. Geosci. 13, 288–295 (2020). https://doi.org/10.1038/s41561-020-0557-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41561-020-0557-6
This article is cited by
-
Sulfate reduction accelerates groundwater arsenic contamination even in aquifers with abundant iron oxides
Nature Water (2023)
-
Arsenic through aquatic trophic levels: effects, transformations and biomagnification—a concise review
Geoscience Letters (2022)
-
Reconstructing Earth’s atmospheric oxygenation history using machine learning
Nature Communications (2022)
-
Effect of oxidation on the release of multiple metals from industrially polluted sediments and synchrotron-based evidence of Cu–S dynamic association
Journal of Soils and Sediments (2022)
-
Variability in groundwater flow and chemistry in the Mekong River alluvial aquifer (Thailand): implications for arsenic and manganese occurrence
Environmental Earth Sciences (2021)