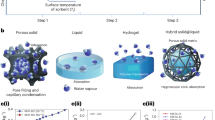
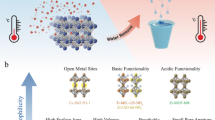
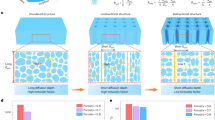
Abstract
The atmosphere contains 13,000 trillion litres of water, and it is a natural resource available anywhere. Sorption-based atmospheric water harvesting (SAWH) is capable of extracting water vapour using sorbent materials across a broad spectrum of relative humidity, opening new avenues to address water scarcity faced by two-thirds of the population of the world. Although substantial progress has been made, there is still a considerable barrier between fundamental research and real-world applications. In this Review, we provide a multiscale perspective for SAWH technologies that can fill existing knowledge gaps across multiple length scales. First, we elucidate water sorption mechanisms at the molecular level, approaches to understanding sorbent materials, and water transport phenomena. With microscopic insights, we bridge materials innovations to device realization, discuss strategies to enhance device-level sorption kinetics and heat transfer performance, and show that a multiscale design and optimization strategy can lead to a new opportunity space towards system thermodynamic limits. Finally, we provide an outlook for the technoeconomic, social and environmental impact of large-scale SAWH as a global water technology. By bridging materials to devices, we envision that this multiscale perspective can guide next-generation SAWH technologies and facilitate a broader impact on society and the environment.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Humphrey, J. H. et al. The potential for atmospheric water harvesting to accelerate household access to safe water. Lancet Planet. Health 4, e91–e92 (2020).
Cotruvo, J. A. 2017 WHO guidelines for drinking water quality: first addendum to the fourth edition. J. Am. Water Work. Assoc. 109, 44–51 (2017).
UNESCO. The United Nations world water development report 2023: partnerships and cooperation for water. UN-Water https://www.unwater.org/publications/un-world-water-development-report-2023 (2023).
Wang, Z. et al. Pathways and challenges for efficient solar-thermal desalination. Sci. Adv. 5, eaax0763 (2019).
Zhang, L. et al. Passive, high-efficiency thermally-localized solar desalination. Energy Environ. Sci. 14, 1771–1793 (2021).
Tu, Y., Wang, R., Zhang, Y. & Wang, J. Progress and expectation of atmospheric water harvesting. Joule 2, 1452–1475 (2018).
Shannon, M. A. et al. Science and technology for water purification in the coming decades. Nature 452, 301–310 (2008).
Kümmerer, K., Dionysiou, D. D., Olsson, O. & Fatta-Kassinos, D. A path to clean water. Science 361, 222–224 (2018).
Kim, H. et al. Water harvesting from air with metal-organic frameworks powered by natural sunlight. Science 356, 430–434 (2017).
Kim, H. et al. Adsorption-based atmospheric water harvesting device for arid climates. Nat. Commun. 9, 1191 (2018).
Fathieh, F. et al. Practical water production from desert air. Sci. Adv. 4, eaat3198 (2018).
Saavedra, J., Doan, H. A., Pursell, C. J., Grabow, L. C. & Chandler, B. D. The critical role of water at the gold-titania interface in catalytic CO oxidation. Science 345, 1599–1602 (2014).
Suguro, T. et al. A hygroscopic nano-membrane coating achieves efficient vapor-fed photocatalytic water splitting. Nat. Commun. 13, 5698 (2022).
Guo, J. et al. Hydrogen production from the air. Nat. Commun. 13, 5046 (2022).
Tatsidjodoung, P., Le Pierrès, N. & Luo, L. A review of potential materials for thermal energy storage in building applications. Renew. Sustain. Energy Rev. 18, 327–349 (2013).
Narayanan, S. et al. Thermal battery for portable climate control. Appl. Energy 149, 104–116 (2015).
Gordeeva, L. G. et al. Metal-organic frameworks for energy conversion and water harvesting: a bridge between thermal engineering and material science. Nano Energy 84, 105946 (2021).
Liu, X. et al. Unusual temperature dependence of water sorption in semicrystalline hydrogels. Adv. Mater. 35, 2211763 (2023).
Poredoš, P. & Wang, R. Sustainable cooling with water generation. Science 380, 458–460 (2023).
Poredoš, P., Shan, H. & Wang, R. Dehumidification with solid hygroscopic sorbents for low-carbon air conditioning. Joule 6, 1390–1393 (2022).
Hanikel, N., Prévot, M. S. & Yaghi, O. M. MOF water harvesters. Nat. Nanotechnol. 15, 348–355 (2020).
Xu, W. & Yaghi, O. M. Metal-organic frameworks for water harvesting from air, anywhere, anytime. ACS Cent. Sci. 6, 1348–1354 (2020).
LaPotin, A. et al. Dual-stage atmospheric water harvesting device for scalable solar-driven water production. Joule 5, 166–182 (2021).
Lord, J. et al. Global potential for harvesting drinking water from air using solar energy. Nature 598, 611–617 (2021).
Xu, J. et al. Ultrahigh solar-driven atmospheric water production enabled by scalable rapid-cycling water harvester with vertically aligned nanocomposite sorbent. Energy Environ. Sci. 14, 5979–5994 (2021).
Li, R. et al. Hybrid hydrogel with high water vapor harvesting capacity for deployable solar-driven atmospheric water generator. Environ. Sci. Technol. 52, 11367–11377 (2018).
Shan, H. et al. Exceptional water production yield enabled by batch-processed portable water harvester in semi-arid climate. Nat. Commun. 13, 5406 (2022).
Hanikel, N. et al. Evolution of water structures in metal-organic frameworks for improved atmospheric water harvesting. Science 374, 454–459 (2021).
Burtch, N. C. et al. In situ visualization of loading-dependent water effects in a stable metal–organic framework. Nat. Chem. 12, 186–192 (2020).
Yang, K. et al. A roadmap to sorption-based atmospheric water harvesting: from molecular sorption mechanism to sorbent design and system optimization. Environ. Sci. Technol. 55, 6542–6560 (2021).
Kalmutzki, M. J., Diercks, C. S. & Yaghi, O. M. Metal–organic frameworks for water harvesting from air. Adv. Mater. 30, 1704304 (2018).
Ball, P. C. & Evans, R. Temperature dependence of gas adsorption on a mesoporous solid: capillary criticality and hysteresis. Langmuir 5, 714–723 (1989).
Morishige, K., Fujii, H., Uga, M. & Kinukawa, D. Capillary critical point of argon, nitrogen, oxygen, ethylene, and carbon dioxide in MCM-41. Langmuir 13, 3494–3498 (1997).
Coasne, B., Gubbins, K. E. & Pellenq, R. J.-M. Temperature effect on adsorption/desorption isotherms for a simple fluid confined within various nanopores. Adsorption 11, 289–294 (2005).
Furukawa, H. et al. Water adsorption in porous metal-organic frameworks and related materials. J. Am. Chem. Soc. 136, 4369–4381 (2014).
Lu, H. et al. Materials engineering for atmospheric water harvesting: progress and perspectives. Adv. Mater. 34, 2110079 (2022).
LaPotin, A., Kim, H., Rao, S. R. & Wang, E. N. Adsorption-based atmospheric water harvesting: impact of material and component properties on system-level performance. Acc. Chem. Res. 52, 1588–1597 (2019).
Graeber, G. et al. Extreme water uptake of hygroscopic hydrogels through maximized swelling‐induced salt loading. Adv. Mater. 36, 2211783 (2023).
Díaz-Marín, C. D. et al. Kinetics of sorption in hygroscopic hydrogels. Nano Lett. 22, 1100–1107 (2022).
Zhao, F. et al. Super moisture-absorbent gels for all-weather atmospheric water harvesting. Adv. Mater. 31, 1806446 (2019).
Chen, G. Thermodynamics of hydrogels for applications in atmospheric water harvesting, evaporation, and desalination. Phys. Chem. Chem. Phys. 24, 12329–12345 (2022).
Lu, H. et al. Tailoring the desorption behavior of hygroscopic gels for atmospheric water harvesting in arid climates. Adv. Mater. 34, 2205344 (2022).
Aleid, S. et al. Salting-in effect of zwitterionic polymer hydrogel facilitates atmospheric water harvesting. ACS Mater. Lett. 4, 511–520 (2022).
Bouklas, N. & Huang, R. Swelling kinetics of polymer gels: comparison of linear and nonlinear theories. Soft Matter 8, 8194–8203 (2012).
Guo, Y. et al. Scalable super hygroscopic polymer films for sustainable moisture harvesting in arid environments. Nat. Commun. 13, 2761 (2022).
Guan, W., Lei, C., Guo, Y., Shi, W. & Yu, G. Hygroscopic‐microgels‐enabled rapid water extraction from arid air. Adv. Mater. 36, 2207786 (2022).
Xu, J. et al. Efficient solar‐driven water harvesting from arid air with metal–organic frameworks modified by hygroscopic salt. Angew. Chem. Int. Ed. 132, 5240–5248 (2020).
Shan, H. et al. High-yield solar-driven atmospheric water harvesting with ultra-high salt content composites encapsulated in porous membrane. Cell Rep. Phys. Sci. 2, 100664 (2021).
Zhao, X. et al. Soft materials by design: unconventional polymer networks give extreme properties. Chem. Rev. 121, 4309–4372 (2021).
Matsumoto, K., Sakikawa, N. & Miyata, T. Thermo-responsive gels that absorb moisture and ooze water. Nat. Commun. 9, 2315 (2018).
Kim, H., Rao, S. R., LaPotin, A., Lee, S. & Wang, E. N. Thermodynamic analysis and optimization of adsorption-based atmospheric water harvesting. Int. J. Heat Mass Transf. 161, 120253 (2020).
Thommes, M. et al. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 87, 1051–1069 (2015).
Liu, X., Wang, X. & Kapteijn, F. Water and metal-organic frameworks: from interaction toward utilization. Chem. Rev. 120, 8303–8377 (2020).
Li, R., Shi, Y., Wu, M., Hong, S. & Wang, P. Photovoltaic panel cooling by atmospheric water sorption–evaporation cycle. Nat. Sustain. 3, 636–643 (2020).
Burtch, N. C., Jasuja, H. & Walton, K. S. Water stability and adsorption in metal-organic frameworks. Chem. Rev. 114, 10575–10612 (2014).
Glueckauf, E. Theory of chromatography. Part 10. Formula for diffusion into spheres and their application to chromatography. Trans. Faraday Soc. 51, 1540–1551 (1955).
Crank, J. The Mathematics of Diffusion (Clarendon Press, 2001).
Davis, M. E. & Davis, R. J. Fundamentals of Chemical Reaction Engineering (McGraw-Hill, 2003).
Sapre, A. V. & Katzer, J. R. Core of chemical reaction engineering: one industrial view. Ind. Eng. Chem. Res. 34, 2202–2225 (1995).
Li, R., Shi, Y., Shi, L., Alsaedi, M. & Wang, P. Harvesting water from air: using anhydrous salt with sunlight. Environ. Sci. Technol. 52, 5398–5406 (2018).
Li, R. & Wang, P. Sorbents, processes and applications beyond water production in sorption-based atmospheric water harvesting. Nat. Water 1, 573–586 (2023).
Kim, H. et al. Characterization of adsorption enthalpy of novel water-stable zeolites and metal-organic frameworks. Sci. Rep. 6, 19097 (2016).
Rieth, A. J., Yang, S., Wang, E. N. & Dincǎ, M. Record atmospheric fresh water capture and heat transfer with a material operating at the water uptake reversibility limit. ACS Cent. Sci. 3, 668–672 (2017).
Simon, C. M. et al. Statistical mechanical model of gas adsorption in porous crystals with dynamic moieties. Proc. Natl Acad. Sci. USA 114, E287–E296 (2017).
Coudert, F. X., Boutin, A., Jeffroy, M., Mellot-Draznieks, C. & Fuchs, A. H. Thermodynamic methods and models to study flexible metal-organic frameworks. ChemPhysChem 12, 247–258 (2011).
Doan, Q. T., Lefèvre, G., Hurisse, O. & Coudert, F. X. Adsorption in complex porous networks with geometrical and chemical heterogeneity. Mol. Simul. 40, 16–24 (2014).
Ng, K. C., Burhan, M., Shahzad, M. W. & Ismail, A. B. A universal isotherm model to capture adsorption uptake and energy distribution of porous heterogeneous surface. Sci. Rep. 7, 10634 (2017).
Grenier, J. et al. Mechanisms of pore formation in hydrogel scaffolds textured by freeze-drying. Acta Biomater. 94, 195–203 (2019).
Singh, M. P., Dhumal, N. R., Kim, H. J., Kiefer, J. & Anderson, J. A. Influence of water on the chemistry and structure of the metal-organic framework Cu3(btc)2. J. Phys. Chem. C 120, 17323–17333 (2016).
DeCoste, J. B. et al. The effect of water adsorption on the structure of the carboxylate containing metal–organic frameworks Cu-BTC, Mg-MOF-74, and UiO-66. J. Mater. Chem. A 1, 11922 (2013).
Ichii, T. et al. Observation of an exotic state of water in the hydrophilic nanospace of porous coordination polymers. Commun. Chem. 3, 16 (2020).
Bae, J., Park, S. H., Moon, D. & Jeong, N. C. Crystalline hydrogen bonding of water molecules confined in a metal-organic framework. Commun. Chem. 5, 51 (2022).
Gul-E-Noor, F. et al. Effects of varying water adsorption on a Cu3(BTC)2 metal-organic framework (MOF) as studied by 1H and 13C solid-state NMR spectroscopy. Phys. Chem. Chem. Phys. 13, 7783–7788 (2011).
Shapiro, Y. E. Structure and dynamics of hydrogels and organogels: an NMR spectroscopy approach. Prog. Polym. Sci. 36, 1184–1253 (2011).
Kärger, J. et al. Pulsed field gradient NMR diffusion measurement in nanoporous materials. Adsorption 27, 453–484 (2021).
Salazar, J. M., Weber, G., Simon, J. M., Bezverkhyy, I. & Bellat, J. P. Characterization of adsorbed water in MIL-53(Al) by FTIR spectroscopy and ab-initio calculations. J. Chem. Phys. 142, 124702 (2015).
Rieth, A. J. et al. Record-setting sorbents for reversible water uptake by systematic anion exchanges in metal-organic frameworks. J. Am. Chem. Soc. 141, 13858–13866 (2019).
Fuchs, A. et al. Water harvesting at the single-crystal level. J. Am. Chem. Soc. 145, 14324–14334 (2023).
Hadjiivanov, K. I. et al. Power of infrared and Raman spectroscopies to characterize metal-organic frameworks and investigate their interaction with guest molecules. Chem. Rev. 121, 1286–1424 (2021).
Fuchs, A. et al. Single crystals heterogeneity impacts the intrinsic and extrinsic properties of metal–organic frameworks. Adv. Mater. 34, 2104530 (2022).
Cho, H. S. et al. Isotherms of individual pores by gas adsorption crystallography. Nat. Chem. 11, 562–570 (2019).
Gong, X. et al. Insights into the structure and dynamics of metal-organic frameworks via transmission electron microscopy. J. Am. Chem. Soc. 142, 17224–17235 (2020).
Li, X. et al. Direct imaging of tunable crystal surface structures of MOF MIL-101 using high-resolution electron microscopy. J. Am. Chem. Soc. 141, 12021–12028 (2019).
Kiyama, R. et al. Nanoscale TEM imaging of hydrogel network architecture. Adv. Mater. 35, 2208902 (2023).
Shen, B. et al. Atomic imaging of zeolite-confined single molecules by electron microscopy. Nature 607, 703–707 (2022).
Shen, B. et al. A single-molecule van der Waals compass. Nature 592, 541–544 (2021).
Xiong, H. et al. In situ imaging of the sorption-induced subcell topological flexibility of a rigid zeolite framework. Science 376, 491–496 (2022).
Cadiau, A. et al. Design of hydrophilic metal organic framework water adsorbents for heat reallocation. Adv. Mater. 27, 4775–4780 (2015).
Wang, S. et al. A robust large-pore zirconium carboxylate metal–organic framework for energy-efficient water-sorption-driven refrigeration. Nat. Energy 3, 985–993 (2018).
Wallace, M., Adams, D. J. & Iggo, J. A. Analysis of the mesh size in a supramolecular hydrogel by PFG-NMR spectroscopy. Soft Matter 9, 5483–5491 (2013).
Tan, K. et al. Water reaction mechanism in metal organic frameworks with coordinatively unsaturated metal ions: MOF-74. Chem. Mater. 26, 6886–6895 (2014).
Rieth, A. J., Hunter, K. M., Dincă, M. & Paesani, F. Hydrogen bonding structure of confined water templated by a metal-organic framework with open metal sites. Nat. Commun. 10, 4771 (2019).
Shi, Y., Ilic, O., Atwater, H. A. & Greer, J. R. All-day fresh water harvesting by microstructured hydrogel membranes. Nat. Commun. 12, 2797 (2021).
Kärger, J. et al. Microimaging of transient guest profiles to monitor mass transfer in nanoporous materials. Nat. Mater. 13, 333–343 (2014).
Valiullin, R., Kärger, J., Cho, K., Choi, M. & Ryoo, R. Dynamics of water diffusion in mesoporous zeolites. Microporous Mesoporous Mater. 142, 236–244 (2011).
Zhang, K. et al. Diffusion of water and ethanol in silicalite crystals synthesized in fluoride media. Microporous Mesoporous Mater. 170, 259–265 (2013).
Watanabe, T. & Sholl, D. S. Accelerating applications of metal-organic frameworks for gas adsorption and separation by computational screening of materials. Langmuir 28, 14114–14128 (2012).
Odoh, S. O., Cramer, C. J., Truhlar, D. G. & Gagliardi, L. Quantum-chemical characterization of the properties and reactivities of metal-organic frameworks. Chem. Rev. 115, 6051–6111 (2015).
Peng, X., Lin, L. C., Sun, W. & Smit, B. Water adsorption in metal-organic frameworks with open-metal sites. AIChE J. 61, 677–687 (2015).
Fischer, M. Water adsorption in SAPO-34: elucidating the role of local heterogeneities and defects using dispersion-corrected DFT calculations. Phys. Chem. Chem. Phys. 17, 25260–25271 (2015).
Skarmoutsos, I., Eddaoudi, M. & Maurin, G. Highly efficient rare-earth-based metal-organic frameworks for water adsorption: a molecular modeling approach. J. Phys. Chem. C 123, 26989–26999 (2019).
Vanduyfhuys, L. et al. QuickFF: a program for a quick and easy derivation of force fields for metal-organic frameworks from ab initio input. J. Comput. Chem. 36, 1015–1027 (2015).
Dürholt, J. P., Fraux, G., Coudert, F. X. & Schmid, R. Ab initio derived force fields for zeolitic imidazolate frameworks: MOF-FF for ZIFs. J. Chem. Theory Comput. 15, 2420–2432 (2019).
Fasano, M. et al. Interplay between hydrophilicity and surface barriers on water transport in zeolite membranes. Nat. Commun. 7, 12762 (2016).
Fei, S., Alizadeh, A., Hsu, W. L., Delaunay, J. J. & Daiguji, H. Analysis of the water adsorption mechanism in metal-organic framework MIL-101(Cr) by molecular simulations. J. Phys. Chem. C 125, 26755–26769 (2021).
Li, Y. et al. H2O adsorption/desorption in MOF-74: ab initio molecular dynamics and experiments. J. Phys. Chem. C 119, 13021–13031 (2015).
Fei, S., Hsu, W.-L., Delaunay, J.-J. & Daiguji, H. Molecular dynamics study of water confined in MIL-101 metal–organic frameworks. J. Chem. Phys. 154, 144503 (2021).
Datar, A., Witman, M. & Lin, L. C. Improving computational assessment of porous materials for water adsorption applications via flat histogram methods. J. Phys. Chem. C 125, 4253–4266 (2021).
Datar, A., Witman, M. & Lin, L. C. Monte Carlo simulations for water adsorption in porous materials: best practices and new insights. AIChE J. 67, e17447 (2021).
Cho, K. H. et al. Rational design of a robust aluminum metal-organic framework for multi-purpose water-sorption-driven heat allocations. Nat. Commun. 11, 5112 (2020).
Choi, J., Lin, L. C. & Grossman, J. C. Role of structural defects in the water adsorption properties of MOF-801. J. Phys. Chem. C 122, 5545–5552 (2018).
Hanikel, N. et al. Rapid cycling and exceptional yield in a metal-organic framework water harvester. ACS Cent. Sci. 5, 1699–1706 (2019).
Nguyen, H. L. et al. Hydrazine-hydrazide-linked covalent organic frameworks for water harvesting. ACS Cent. Sci. 8, 926–932 (2022).
Krajnc, A. et al. Superior performance of microporous aluminophosphate with LTA topology in solar-energy storage and heat reallocation. Adv. Energy Mater. 7, 1601815 (2017).
Mittal, H., Al Alili, A. & Alhassan, S. M. Capturing water vapors from atmospheric air using superporous gels. Sci. Rep. 12, 5626 (2022).
Garzón-Tovar, L., Pérez-Carvajal, J., Imaz, I. & Maspoch, D. Composite salt in porous metal-organic frameworks for adsorption heat transformation. Adv. Funct. Mater. 27, 1606424 (2017).
Entezari, A., Ejeian, M. & Wang, R. Super atmospheric water harvesting hydrogel with alginate chains modified with binary salts. ACS Mater. Lett. 2, 471–477 (2020).
Chen, Z. et al. Study of the scale-up effect on the water sorption performance of MOF materials. ACS Mater. Au 3, 43–54 (2023).
Ejeian, M. & Wang, R. Z. Adsorption-based atmospheric water harvesting. Joule 5, 1678–1703 (2021).
Bagi, S., Wright, A. M., Oppenheim, J., Dincǎ, M. & Román-Leshkov, Y. Accelerated synthesis of a Ni2Cl2(BTDD) metal-organic framework in a continuous flow reactor for atmospheric water capture. ACS Sustain. Chem. Eng. 9, 3996–4003 (2021).
Zheng, Z. et al. High-yield, green and scalable methods for producing MOF-303 for water harvesting from desert air. Nat. Protoc. 18, 136–156 (2023).
Yaghi, O. M., Kalmutzki, M. J. & Diercks, C. S. Introduction to Reticular Chemistry (Wiley, 2019).
Zheng, Z., Hanikel, N., Lyu, H. & Yaghi, O. M. Broadly tunable atmospheric water harvesting in multivariate metal-organic frameworks. J. Am. Chem. Soc. 144, 22669–22675 (2022).
Ji, Z., Wang, H., Canossa, S., Wuttke, S. & Yaghi, O. M. Pore chemistry of metal–organic frameworks. Adv. Funct. Mater. 30, 2000238 (2020).
Sun, Y. et al. Tunable LiCl@UiO-66 composites for water sorption-based heat transformation applications. J. Mater. Chem. A 8, 13364–13375 (2020).
Zhou, X., Lu, H., Zhao, F. & Yu, G. Atmospheric water harvesting: a review of material and structural designs. ACS Mater. Lett. 2, 671–684 (2020).
Fatouh, M., Metwally, M. N., Helali, A. B. & Shedid, M. H. Herbs drying using a heat pump dryer. Energy Convers. Manag. 47, 2629–2643 (2006).
Chua, K. J., Chou, S. K. & Yang, W. M. Advances in heat pump systems: a review. Appl. Energy 87, 3611–3624 (2010).
Kim, J., Park, K., Yang, D. R. & Hong, S. A comprehensive review of energy consumption of seawater reverse osmosis desalination plants. Appl. Energy 254, 113652 (2019).
Legrand, U., Girard-Lauriault, P. L., Meunier, J. L., Boudreault, R. & Tavares, J. R. Experimental and theoretical assessment of water sorbent kinetics. Langmuir 38, 2651–2659 (2022).
Díaz-Marín, C. D. et al. Heat and mass transfer in hygroscopic hydrogels. Int. J. Heat Mass Transf. 195, 123103 (2022).
Feng, Y., Ge, T., Chen, B., Zhan, G. & Wang, R. A regulation strategy of sorbent stepwise position for boosting atmospheric water harvesting in arid area. Cell Rep. Phys. Sci. 2, 100561 (2021).
El Fil, B., Li, X., Díaz-Marín, C. D., Zhang, L. & Jacobucci, C. L. Significant enhancement of sorption kinetics via boiling-assisted channel templating. Cell Rep. Phys. Sci. 4, 101549 (2023).
Wilson, C. T. et al. Design considerations for next-generation sorbent-based atmospheric water-harvesting devices. Device 1, 100052 (2023).
Zhao, L. et al. Harnessing heat beyond 200 °C from unconcentrated sunlight with nonevacuated transparent aerogels. ACS Nano 13, 7508–7516 (2019).
Li, A. C. et al. Thermodynamic limits of atmospheric water harvesting with temperature-dependent adsorption. Appl. Phys. Lett. 121, 164102 (2022).
Wang, J. Y., Wang, R. Z., Tu, Y. D. & Wang, L. W. Universal scalable sorption-based atmosphere water harvesting. Energy 165, 387–395 (2018).
Feng, Y., Wang, R. & Ge, T. Pathways to energy-efficient water production from the atmosphere. Adv. Sci. 9, 2204508 (2022).
US Annual Solar GHI. The National Renewable Energy Laboratory https://www.nrel.gov/gis/solar.html (2018).
Haechler, I. et al. Exploiting radiative cooling for uninterrupted 24-hour water harvesting from the atmosphere. Sci. Adv. 7, eabf3978 (2021).
Bai, S. et al. Adsorption-based atmospheric water harvesting by passive radiative condensers for continuous decentralized water production. Appl. Therm. Eng. 225, 120163 (2023).
Xu, Z. et al. Ultrahigh-efficiency desalination via a thermally-localized multistage solar still. Energy Environ. Sci. 13, 830–839 (2020).
Zhang, L. et al. Modeling and performance analysis of high-efficiency thermally-localized multistage solar stills. Appl. Energy 266, 114864 (2020).
Thomas, T. M., Sinha Mahapatra, P., Ganguly, R. & Tiwari, M. K. Preferred mode of atmospheric water vapor condensation on nanoengineered surfaces: dropwise or filmwise? Langmuir 39, 5396–5407 (2023).
Wang, J., Hua, L., Li, C. & Wang, R. Atmospheric water harvesting: critical metrics and challenges. Energy Environ. Sci. 15, 4867–4871 (2022).
Zhang, Y. & Tan, S. C. Best practices for solar water production technologies. Nat. Sustain. 5, 554–556 (2022).
DeSantis, D. et al. Techno-economic analysis of metal–organic frameworks for hydrogen and natural gas storage. Energy Fuels 31, 2024–2032 (2017).
Zechman Berglund, E. et al. Water and wastewater systems and utilities: challenges and opportunities during the COVID-19 pandemic. J. Water Resour. Plan. Manag. 147, 02521001 (2021).
Sapkota, M. et al. An overview of hybrid water supply systems in the context of urban water management: challenges and opportunities. Water 7, 153–174 (2015).
Xu, J. et al. Near-zero-energy smart battery thermal management enabled by sorption energy harvesting from air. ACS Cent. Sci. 6, 1542–1554 (2020).
Zhao, F., Guo, Y., Zhou, X., Shi, W. & Yu, G. Materials for solar-powered water evaporation. Nat. Rev. Mater. 5, 388–401 (2020).
Aeschlimann, M., Li, G., Kanji, Z. A. & Mitrano, D. M. Potential impacts of atmospheric microplastics and nanoplastics on cloud formation processes. Nat. Geosci. 15, 967–975 (2022).
Trenberth, K. E. Atmospheric moisture recycling: role of advection and local evaporation. J. Clim. 12, 1368–1381 (1999).
Konapala, G., Mishra, A. K., Wada, Y. & Mann, M. E. Climate change will affect global water availability through compounding changes in seasonal precipitation and evaporation. Nat. Commun. 11, 3044 (2020).
Fanning, A. F. & Weaver, A. J. An atmospheric energy-moisture balance model: climatology, interpentadal climate change, and coupling to an ocean general circulation model. J. Geophys. Res. Atmos. 101, 15111–15128 (1996).
March, C. & Page, S. E. E. L. Atmospheric CO2: principal control knob governing Earth’s temperature. Science 330, 356–359 (2010).
NASA Science Editorial Team. Steamy relationships: how atmospheric water vapor amplifies Earth’s greenhouse effect. NASA https://science.nasa.gov/earth/climate-change/steamy-relationships-how-atmospheric-water-vapor-amplifies-earths-greenhouse-effect/ (2022).
Manabe, S. & Stouffer, R. J. Sensitivity of a global climate model to an increase of CO2 concentration in the atmosphere. J. Geophys. Res. Ocean. 85, 5529–5554 (1980).
Manabe, S. & Wetherald, R. T. Thermal equilibrium of the atmosphere with a given distribution of relative humidity. J. Atmos. Sci. 24, 241–259 (1967).
Held, I. M. & Soden, B. J. Water vapor feedback and global warming. Annu. Rev. Energy Environ. 25, 441–475 (2000).
Acknowledgements
The authors gratefully acknowledge support received from the Defense Advanced Research Projects Agency (DARPA) Atmospheric Water Extraction (AWE) programme under contract HR001120S0014 with S. Cohen as programme manager. Y.Z. acknowledges funding support from MIT MathWorks Engineering fellowship.
Author information
Authors and Affiliations
Contributions
Y.Z. and L.Z. contributed equally to this work. Y.Z., L.Z. and E.N.W. conceptualized the manuscript. Y.Z., L.Z., X.L., B.E.F. and C.D.D.-M. researched data and performed analysis for the article. L.Z. and Y.Z. conceived and illustrated the figures and tables. All authors contributed to the discussion of content, writing, and editing of the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Materials thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhong, Y., Zhang, L., Li, X. et al. Bridging materials innovations to sorption-based atmospheric water harvesting devices. Nat Rev Mater (2024). https://doi.org/10.1038/s41578-024-00665-2
Accepted:
Published:
DOI: https://doi.org/10.1038/s41578-024-00665-2