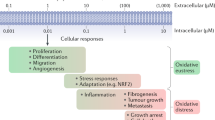
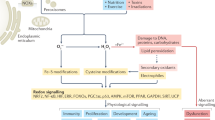
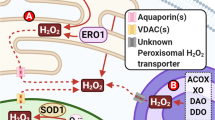
Abstract
Oxidation–reduction (redox) reactions are central to the existence of life. Reactive species of oxygen, nitrogen and sulfur mediate redox control of a wide range of essential cellular processes. Yet, excessive levels of oxidants are associated with ageing and many diseases, including cardiological and neurodegenerative diseases, and cancer. Hence, maintaining the fine-tuned steady-state balance of reactive species production and removal is essential. Here, we discuss new insights into the dynamic maintenance of redox homeostasis (that is, redox homeodynamics) and the principles underlying biological redox organization, termed the ‘redox code’. We survey how redox changes result in stress responses by hormesis mechanisms, and how the lifelong cumulative exposure to environmental agents, termed the ‘exposome’, is communicated to cells through redox signals. Better understanding of the molecular and cellular basis of redox biology will guide novel redox medicine approaches aimed at preventing and treating diseases associated with disturbed redox regulation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Walsh, C. T., Tu, B. P. & Tang, Y. Eight kinetically stable but thermodynamically activated molecules that power cell metabolism. Chem. Rev. 118, 1460–1494 (2018).
Jacob, C., Giles, G. I., Giles, N. M. & Sies, H. Sulfur and selenium: the role of oxidation state in protein structure and function. Angew. Chem. Int. Ed. Engl. 42, 4742–4758 (2003).
Lennicke, C. & Cochemé, H. M. Redox metabolism: ROS as specific molecular regulators of cell signaling and function. Mol. Cell 81, 3691–3707 (2021).
Sies, H. et al. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 23, 499–515 (2022). This ‘Expert Recommendation’ addresses key questions regarding the impact of oxidants on physiology and their contribution to disease.
Thannickal, V. J. & Fanburg, B. L. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell Mol. Physiol. 279, L1005–L1028 (2000).
D’Autreaux, B. & Toledano, M. B. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 8, 813–824 (2007). This article describes fundamental perspectives on reactive oxygen species signalling.
Butterfield, D. A. & Perluigi, M. Redox proteomics: a key tool for new insights into protein modification with relevance to disease. Antioxid. Redox Signal. 26, 277–279 (2017).
Murphy, M. P. et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 4, 651–662 (2022).
Sies, H. & Jones, D. P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 21, 363–383 (2020).
Radi, R. Oxygen radicals, nitric oxide, and peroxynitrite: redox pathways in molecular medicine. Proc. Natl Acad. Sci. USA 115, 5839–5848 (2018).
Lundberg, J. O. & Weitzberg, E. Nitric oxide signaling in health and disease. Cell 185, 2853–2878 (2022).
Cirino, G., Szabo, C. & Papapetropoulos, A. Physiological roles of hydrogen sulfide in mammalian cells, tissues, and organs. Physiol. Rev. 103, 31–276 (2023).
Parvez, S., Long, M. J. C., Poganik, J. R. & Aye, Y. Redox signaling by reactive electrophiles and oxidants. Chem. Rev. 118, 8798–8888 (2018). This is a comprehensive review of the signalling role of electrophiles and oxidants in biology.
Rabbani, N. & Thornalley, P. J. Protein glycation — biomarkers of metabolic dysfunction and early-stage decline in health in the era of precision medicine. Redox Biol. 42, 101920 (2021).
Noctor, G. & Foyer, C. H. Intracellular redox compartmentation and ROS-related communication in regulation and signaling. Plant Physiol. 171, 1581–1592 (2016).
Mittler, R., Zandalinas, S. I., Fichman, Y. & Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 23, 663–679 (2022).
Dietz, K. J. & Vogelsang, L. A general concept of quantitative abiotic stress sensing. Trends Plant Sci. 23, 237–246 (2023).
Sies, H. (ed.) Oxidative Stress: Eustress and Distress 1–844 (Academic Press, 2020).
Wild, C. P. The exposome: from concept to utility. Int. J. Epidemiol. 41, 24–32 (2012).
Xiao, W. & Loscalzo, J. Metabolic responses to reductive stress. Antioxid. Redox Signal. 32, 1330–1347 (2020).
Hanschmann, E. M., Godoy, J. R., Berndt, C., Hudemann, C. & Lillig, C. H. Thioredoxins, glutaredoxins, and peroxiredoxins — molecular mechanisms and health significance: from cofactors to antioxidants to redox signaling. Antioxid. Redox Signal. 19, 1539–1605 (2013).
Jones, D. P. & Sies, H. The redox code. Antioxid. Redox Signal. 23, 734–746 (2015). This article introduces the ‘redox code’ as a set of fundamental principles of organization of biological redox reactions.
Prigogine, I. Time, structure, and fluctuations. Science 201, 777–785 (1978).
Sies, H. Oxidative eustress: on constant alert for redox homeostasis. Redox Biol. 41, 101867 (2021).
Kondadi, A. K. et al. Cristae undergo continuous cycles of membrane remodelling in a MICOS-dependent manner. EMBO Rep. 21, e49776 (2020).
Billman, G. E. Homeostasis: the underappreciated and far too often ignored central organizing principle of physiology. Front. Physiol. 11, 200 (2020).
Lloyd, D., Aon, M. A. & Cortassa, S. Why homeodynamics, not homeostasis? Scientific World J. 1, 133–145 (2001).
Xiong, L. I. & Garfinkel, A. Are physiological oscillations physiological? J. Physiol. https://doi.org/10.1113/JP285015 (2023).
Brash, D. E. Rethinking causation for data-intensive biology: constraints, cancellations, and quantized organisms: causality in complex organisms is sculpted by constraints rather than instigators, with outcomes perhaps better described by quantized patterns than rectilinear pathways. Bioessays 42, e1900135 (2020).
Sies, H., Berndt, C. & Jones, D. P. Oxidative stress. Annu. Rev. Biochem. 86, 715–748 (2017). This article discusses the concept of oxidative stress, physiological (eustress) and supraphysiological (distress).
Lushchak, V. I. & Storey, K. B. Oxidative stress concept updated: definitions, classifications and regulatory pathways implicated. EXCLI J. 20, 956–967 (2021).
Rattan, S. I. Molecular gerontology: from homeodynamics to hormesis. Curr. Pharm. Des. 20, 3036–3039 (2014).
Alleman, R. J., Katunga, L. A., Nelson, M. A., Brown, D. A. & Anderson, E. J. The ‘Goldilocks Zone’ from a redox perspective — adaptive vs. deleterious responses to oxidative stress in striated muscle. Front. Physiol. 5, 358 (2014).
Ursini, F., Maiorino, M. & Forman, H. J. Redox homeostasis: the golden mean of healthy living. Redox Biol. 8, 205–215 (2016).
Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: oxidative eustress. Redox Biol. 11, 613–619 (2017).
Winterbourn, C. C. Are free radicals involved in thiol-based redox signaling? Free Radic. Biol. Med. 80, 164–170 (2015).
Holmström, K. M. & Finkel, T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 15, 411–421 (2014).
Brigelius-Flohé, R. & Flohé, L. Basic principles and emerging concepts in the redox control of transcription factors. Antioxid. Redox Signal. 15, 2335–2381 (2011). Together with Marinho et al. (2014), this comprehensive review discusses redox control of transcription factors.
Marinho, H. S., Real, C., Cyrne, L., Soares, H. & Antunes, F. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol. 2, 535–562 (2014). Together with Brigelius-Flohé and Flohé (2011), this comprehensive review discusses redox control of transcription factors.
Jose, E. et al. Temporal coordination of the transcription factor response to H2O2 stress. Nat. Commun. 15, 3440 (2024).
Müller, N. et al. Reactive oxygen species differentially modulate the metabolic and transcriptomic response of endothelial cells. Antioxidants 11, 434 (2022).
Suzuki, T. & Yamamoto, M. Stress-sensing mechanisms and the physiological roles of the Keap1-Nrf2 system during cellular stress. J. Biol. Chem. 292, 16817–16824 (2017).
Yamamoto, M., Kensler, T. W. & Motohashi, H. The KEAP1-nrf2 system: a thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol. Rev. 98, 1169–1203 (2018). A comprehensive review of the mechanism, biology and medical aspects of the KEAP1–NRF2 system that regulates responses to oxidative and electrophilic challenges.
Cuadrado, A. et al. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat. Rev. Drug Discov. 18, 295–317 (2019).
Calabrese, E. J. & Kozumbo, W. J. The hormetic dose–response mechanism: Nrf2 activation. Pharmacol. Res. 167, 105526 (2021). This article describes the pivotal role of the NRF2 system in hormetic responses.
Agathokleous, E. & Calabrese, E. J. Hormesis: a general biological principle. Chem. Res. Toxicol. 35, 547–549 (2022).
Mattson, M. P. & Leak, R. K. The hormesis principle of neuroplasticity and neuroprotection. Cell Metab. 36, 315–337 (2024).
Baird, L. et al. A NRF2-induced secretory phenotype activates immune surveillance to remove irreparably damaged cells. Redox Biol. 66, 102845 (2023).
Agarwal, S. & Ganesh, S. Perinuclear mitochondrial clustering, increased ROS levels, and HIF1 are required for the activation of HSF1 by heat stress. J. Cell Sci. 133, jcs245589 (2020).
Al-Mehdi, A. B. et al. Perinuclear mitochondrial clustering creates an oxidant-rich nuclear domain required for hypoxia-induced transcription. Sci. Signal. 5, ra47 (2012).
Cyran, A. M. & Zhitkovich, A. HIF1, HSF1, and NRF2: oxidant-responsive trio raising cellular defenses and engaging immune system. Chem. Res. Toxicol. 35, 1690–1700 (2022).
Liu, S. H., Qiu, Y., Xiang, R. & Huang, P. Characterization of H2O2-induced alterations in global transcription of mRNA and lncRNA. Antioxidants (Basel) 11, 495 (2022).
D’Souza, L. C. et al. Oxidative stress and cancer development: are noncoding RNAs the missing links? Antioxid. Redox Signal. 33, 1209–1229 (2020).
Zheng, F. et al. The HIF-1alpha antisense long non-coding RNA drives a positive feedback loop of HIF-1alpha mediated transactivation and glycolysis. Nat. Commun. 12, 1341 (2021).
Ji, Q., Zong, X., Mao, Y. & Qian, S. B. A heat shock-responsive lncRNA heat acts as a HSF1-directed transcriptional brake via m(6)A modification. Proc. Natl Acad. Sci. USA 118, e2102175118 (2021).
Weiss-Sadan, T. et al. NRF2 activation induces NADH-reductive stress, providing a metabolic vulnerability in lung cancer. Cell Metab. 35, 722 (2023).
Manford, A. G. et al. A cellular mechanism to detect and alleviate reductive stress. Cell 183, 46–61.e21 (2020).
Galluzzi, L., Yamazaki, T. & Kroemer, G. Linking cellular stress responses to systemic homeostasis. Nat. Rev. Mol. Cell Biol. 19, 731–745 (2018).
Costa-Mattioli, M. & Walter, P. The integrated stress response: from mechanism to disease. Science 368, eaat5314 (2020).
Hetz, C., Zhang, K. & Kaufman, R. J. Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol. 21, 421–438 (2020).
Lu, H., Yang, M. & Zhou, Q. Reprogramming transcription after DNA damage: recognition, response, repair, and restart. Trends Cell Biol. 33, 682–694 (2023).
Gonzalez-Quiroz, M. et al. When endoplasmic reticulum proteostasis meets the DNA damage response. Trends Cell Biol. 30, 881–891 (2020).
He, F., Ru, X. & Wen, T. NRF2, a transcription factor for stress response and beyond. Int. J. Mol. Sci. 21, 4777 (2020).
Johansson, K. et al. Cross talk in HEK293 cells between Nrf2, HIF, and NF-kappaB activities upon challenges with redox therapeutics characterized with single-cell resolution. Antioxid. Redox Signal. 26, 229–246 (2017).
Meng, J., Lv, Z., Wang, Y. & Chen, C. Identification of the redox-stress signaling threshold (RST): increased RST helps to delay aging in C. elegans. Free Radic. Biol. Med. 178, 54–58 (2022).
Pallepati, P. & Averill-Bates, D. A. Activation of ER stress and apoptosis by hydrogen peroxide in HeLa cells: protective role of mild heat preconditioning at 40 °C. Biochim. Biophys. Acta 1813, 1987–1999 (2011).
Pospelova, T. V. et al. Pseudo-DNA damage response in senescent cells. Cell Cycle 8, 4112–4118 (2009).
Halvey, P. J. et al. Compartmental oxidation of thiol-disulphide redox couples during epidermal growth factor signalling. Biochem. J. 386, 215–219 (2005).
Bergerhausen, L. et al. Extracellular redox regulation of α7β integrin-mediated cell migration is signaled via a dominant thiol-switch. Antioxidants 9, 227 (2020).
Nordzieke, D. E. & Medraño-Fernandez, I. The plasma membrane: a platform for intra- and intercellular redox signaling. Antioxidants 7, 168 (2018). This article provides a comprehensive overview of the plasma membrane as redox signalling platform.
Petersen, S. V., Poulsen, N. B., Linneberg Matthiesen, C. & Vilhardt, F. Novel and converging ways of NOX2 and SOD3 in trafficking and redox signaling in macrophages. Antioxidants 10, 172 (2021).
Bienert, G. P. & Chaumont, F. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim. Biophys. Acta 1840, 1596–1604 (2014). This paper is a seminal work on the role of some aquaporins as peroxiporins.
Mishina, N. M. et al. Does cellular hydrogen peroxide diffuse or act locally? Antioxid. Redox Signal. 14, 1–7 (2011).
Oakley, F. D., Abbott, D., Li, Q. & Engelhardt, J. F. Signaling components of redox active endosomes: the redoxosomes. Antioxid. Redox Signal. 11, 1313–1333 (2009). These findings shaped the concept of redox-active endosomes as redoxosomes.
Spencer, N. Y. & Engelhardt, J. F. The basic biology of redoxosomes in cytokine-mediated signal transduction and implications for disease-specific therapies. Biochemistry 53, 1551–1564 (2014).
Balta, E., Kramer, J. & Samstag, Y. Redox regulation of the actin cytoskeleton in cell migration and adhesion: on the way to a spatiotemporal view. Front. Cell Dev. Biol. 8, 618261 (2020).
Rouyère, C., Serrano, T., Fremont, S. & Echard, A. Oxidation and reduction of actin: origin, impact in vitro and functional consequences in vivo. Eur. J. Cell Biol. 101, 151249 (2022).
Kang, M. I., Kobayashi, A., Wakabayashi, N., Kim, S. G. & Yamamoto, M. Scaffolding of Keap1 to the actin cytoskeleton controls the function of Nrf2 as key regulator of cytoprotective phase 2 genes. Proc. Natl Acad. Sci. USA 101, 2046–2051 (2004).
Valdivia, A., Duran, C. & San Martin, A. The role of nox-mediated oxidation in the regulation of cytoskeletal dynamics. Curr. Pharm. Des. 21, 6009–6022 (2015).
Perez-Sala, D. & Quinlan, R. A. The redox-responsive roles of intermediate filaments in cellular stress detection, integration and mitigation. Curr. Opin. Cell Biol. 86, 102283 (2023).
Walker, C. L., Pomatto, L. C. D., Tripathi, D. N. & Davies, K. J. A. Redox regulation of homeostasis and proteostasis in peroxisomes. Physiol. Rev. 98, 89–115 (2018).
Fujiki, Y., Okumoto, K., Honsho, M. & Abe, Y. Molecular insights into peroxisome homeostasis and peroxisome biogenesis disorders. Biochim. Biophys. Acta Mol. Cell Res. 1869, 119330 (2022).
Sandalio, L. M., Collado-Arenal, A. M. & Romero-Puertas, M. C. Deciphering peroxisomal reactive species interactome and redox signalling networks. Free Radic. Biol. Med. 197, 58–70 (2023).
Lismont, C. et al. Peroxisome-derived hydrogen peroxide modulates the sulfenylation profiles of key redox signaling proteins in Flp-In T-REx 293 cells. Front. Cell Dev. Biol. 10, 888873 (2022). This work demonstrates the role of peroxisomal hydrogen peroxide in intracellular redox communication.
Okumoto, K. et al. The peroxisome counteracts oxidative stresses by suppressing catalase import via Pex14 phosphorylation. eLife 9, e55896 (2020).
Dubreuil, M. M. et al. Systematic identification of regulators of oxidative stress reveals non-canonical roles for peroxisomal import and the pentose phosphate pathway. Cell Rep. 30, 1417–1433 (2020).
Di Cara, F., Savary, S., Kovacs, W. J., Kim, P. & Rachubinski, R. A. The peroxisome: an up-and-coming organelle in immunometabolism. Trends Cell Biol. 33, 70–86 (2023).
Wenzel, E. M., Elfmark, L. A., Stenmark, H. & Raiborg, C. ER as master regulator of membrane trafficking and organelle function. J. Cell Biol. 221, e202205135 (2022).
Rashdan, N. A. & Pattillo, C. B. Hydrogen peroxide in the ER: a tale of triage. Redox Biol. 28, 101358 (2020).
Kirstein, J. et al. Proteotoxic stress and ageing triggers the loss of redox homeostasis across cellular compartments. EMBO J. 34, 2334–2349 (2015).
Jacobs, L. J. & Riemer, J. Maintenance of small molecule redox homeostasis in mitochondria. FEBS Lett. 597, 205–223 (2022).
Shadel, G. S. & Horvath, T. L. Mitochondrial ROS signaling in organismal homeostasis. Cell 163, 560–569 (2015).
Kondadi, A. K. & Reichert, A. S. Mitochondrial dynamics at different levels: from cristae dynamics to interorganellar cross talk. Annu. Rev. Biophys. https://doi.org/10.1146/annurev-biophys-030822-020736 (2024).
Chakrabarty, R. P. & Chandel, N. S. Beyond ATP, new roles of mitochondria. Biochem 44, 2–8 (2022).
Monzel, A. S., Enriquez, J. A. & Picard, M. Multifaceted mitochondria: moving mitochondrial science beyond function and dysfunction. Nat. Metab. 5, 546–562 (2023).
Popov, L. D. Mitochondria as intracellular signalling organelles. An update. Cell Signal. 109, 110794 (2023).
Murphy, M. P. How mitochondria produce reactive oxygen species. Biochem. J. 417, 1–13 (2009).
Hoehne, M. N. et al. Spatial and temporal control of mitochondrial H2O2 release in intact human cells. EMBO J. 41, e109169 (2022).
Koren, S. A. et al. All-optical spatiotemporal mapping of ROS dynamics across mitochondrial microdomains in situ. Nat. Commun. 14, 6036 (2023).
Grayson, C. & Mailloux, R. J. Coenzyme Q(10) and nicotinamide nucleotide transhydrogenase: sentinels for mitochondrial hydrogen peroxide signaling. Free Radic. Biol. Med. 208, 260–271 (2023).
Mills, E. L. et al. Cysteine 253 of UCP1 regulates energy expenditure and sex-dependent adipose tissue inflammation. Cell Metab. 34, 140–157 (2022).
Pei, J. F. et al. Diurnal oscillations of endogenous H(2)O(2) sustained by p66(Shc) regulate circadian clocks. Nat. Cell Biol. 21, 1553–1564 (2019). This work demonstrates the circadian rhythm of endogenously produced hydrogen peroxide.
Funato, Y. et al. Nucleoredoxin, a novel thioredoxin family member involved in cell growth and differentiation. Antioxid. Redox Signal. 9, 1035–1057 (2007).
Idelfonso-Garcia, O. G. et al. Is nucleoredoxin a master regulator of cellular redox homeostasis? Its implication in different pathologies. Antioxidants 11, 670 (2022).
Seifermann, M. & Epe, B. Oxidatively generated base modifications in DNA: not only carcinogenic risk factor but also regulatory mark? Free Radic. Biol. Med. 107, 258–265 (2017).
Fleming, A. M. & Burrows, C. J. Chemistry of ROS-mediated oxidation to the guanine base in DNA and its biological consequences. Int. J. Radiat. Biol. 98, 452–460 (2022).
Hahm, J. Y., Park, J., Jang, E. S. & Chi, S. W. 8-Oxoguanine: from oxidative damage to epigenetic and epitranscriptional modification. Exp. Mol. Med. 54, 1626–1642 (2022).
Perillo, B., Tramontano, A., Pezone, A. & Migliaccio, A. LSD1: more than demethylation of histone lysine residues. Exp. Mol. Med. 52, 1936–1947 (2020).
Leisegang, M. S., Schröder, K. & Brandes, R. P. Redox regulation and noncoding RNAs. Antioxid. Redox Signal. 29, 793–812 (2018). This paper shows a comprehensive overview of the role of non-coding RNAs in redox regulation.
Lettieri-Barbato, D., Aquilano, K., Punziano, C., Minopoli, G. & Faraonio, R. MicroRNAs, long non-coding RNAs, and circular RNAs in the redox control of cell senescence. Antioxidants (Basel) 11, 480 (2022).
Ciesielska, S., Slezak-Prochazka, I., Bil, P. & Rzeszowska-Wolny, J. Micro RNAs in regulation of cellular redox homeostasis. Int. J. Mol. Sci. 22, 6022 (2021).
Zhang, Y. et al. Circular RNAs in the regulation of oxidative stress. Front. Pharmacol. 12, 697903 (2021).
Mercatelli, N. et al. MiR-23-TrxR1 as a novel molecular axis in skeletal muscle differentiation. Sci. Rep. 7, 7219 (2017).
Fuschi, P. et al. Central role of the p53 pathway in the noncoding-RNA response to oxidative stress. Aging 9, 2559–2586 (2017).
Prinz, W. A., Toulmay, A. & Balla, T. The functional universe of membrane contact sites. Nat. Rev. Mol. Cell Biol. 21, 7–24 (2020).
Nieto-Garai, J. A. et al. Super-resolution microscopy to study interorganelle contact sites. Int. J. Mol. Sci. 23, 15354 (2022).
Fuentes-Lemus, E. & Davies, M. J. Effect of crowding, compartmentalization and nanodomains on protein modification and redox signaling — current state and future challenges. Free Radic. Biol. Med. 196, 81–92 (2023).
Yoboue, E. D., Sitia, R. & Simmen, T. Redox crosstalk at endoplasmic reticulum (ER) membrane contact sites (MCS) uses toxic waste to deliver messages. Cell Death Dis. 9, 331–0033 (2018). This paper describes the concept of endoplasmic reticulum–mitochondria–peroxisomes crosstalk: ‘redox triangle’.
Gordaliza-Alaguero, I., Canto, C. & Zorzano, A. Metabolic implications of organelle–mitochondria communication. EMBO Rep. 20, e47928 (2019).
Resende, R., Fernandes, T., Pereira, A. C., Marques, A. P. & Pereira, C. F. Endoplasmic reticulum–mitochondria contacts modulate reactive oxygen species-mediated signaling and oxidative stress in brain disorders: the key role of sigma-1 receptor. Antioxid. Redox Signal. 37, 758–780 (2022).
Bestetti, S. et al. Human aquaporin-11 guarantees efficient transport of H(2)O(2) across the endoplasmic reticulum membrane. Redox Biol. 28, 101326 (2020).
Sorrentino, I., Galli, M., Medrano-Fernandez, I. & Sitia, R. Transfer of H2O2 from mitochondria to the endoplasmic reticulum via aquaporin-11. Redox Biol. 55, 102410 (2022).
Fox, A. R. et al. Plasma membrane aquaporins interact with the endoplasmic reticulum resident VAP27 proteins at ER-PM contact sites and endocytic structures. N. Phytol. 228, 973–988 (2020).
Azad, A. K. et al. Human aquaporins: functional diversity and potential roles in infectious and non-infectious diseases. Front. Genet. 12, 654865 (2021).
Balderas, P. M. D. Mitochondria–plasma membrane interactions and communication. J. Biol. Chem. 297, 101164 (2021).
Upham, B. L. & Trosko, J. E. Oxidative-dependent integration of signal transduction with intercellular gap junctional communication in the control of gene expression. Antioxid. Redox Signal. 11, 297–307 (2009).
Bücher, T. et al. State of oxidation–reduction and state of binding in the cytosolic NADH-system as disclosed by equilibration with extracellular lactate-pyruvate in hemoglobin-free perfused rat liver. Eur. J. Biochem. 27, 301–317 (1972).
Hassinen, I. E. Signaling and regulation through the NAD(+) and NADP(+) networks. Antioxid. Redox Signal. 30, 857–874 (2019).
Lucaciu, S. A., Leighton, S. E., Hauser, A., Yee, R. & Laird, D. W. Diversity in connexin biology. J. Biol. Chem. 299, 105263 (2023).
Zhang, K. et al. The mutual interplay of redox signaling and connexins. J. Mol. Med. 99, 933–941 (2021).
van Niel, G. et al. Challenges and directions in studying cell–cell communication by extracellular vesicles. Nat. Rev. Mol. Cell Biol. 23, 369–382 (2022). This paper shows a comprehensive review on the role of extracellular vesicles in cell–cell communication.
Dixson, A. C., Dawson, T. R., Di Vizio, D. & Weaver, A. M. Context-specific regulation of extracellular vesicle biogenesis and cargo selection. Nat. Rev. Mol. Cell Biol. 24, 454–476 (2023).
Zhang, W. J., Liu, R., Chen, Y. H., Wang, M. H. & Du, J. Crosstalk between oxidative stress and exosomes. Oxid. Med. Cell Longev. 2022, 3553617 (2022).
Hervera, A. et al. Reactive oxygen species regulate axonal regeneration through the release of exosomal NADPH oxidase 2 complexes into injured axons. Nat. Cell Biol. 20, 307–319 (2018).
Clarke-Bland, C. E., Bill, R. M. & Devitt, A. Emerging roles for AQP in mammalian extracellular vesicles. Biochim. Biophys. Acta Biomembr. 1864, 183826 (2022).
Fichman, Y., Rowland, L., Oliver, M. J. & Mittler, R. ROS are evolutionary conserved cell-to-cell stress signals. Proc. Natl Acad. Sci. USA 120, e2305496120 (2023).
Saraswathibhatla, A., Indana, D. & Chaudhuri, O. Cell–extracellular matrix mechanotransduction in 3D. Nat. Rev. Mol. Cell Biol. 24, 495–516 (2023).
Taniguchi, N. et al. Glyco-redox, a link between oxidative stress and changes of glycans: lessons from research on glutathione, reactive oxygen and nitrogen species to glycobiology. Arch. Biochem. Biophys. 595, 72–80 (2016).
Khoder-Agha, F. & Kietzmann, T. The glyco-redox interplay: principles and consequences on the role of reactive oxygen species during protein glycosylation. Redox Biol. 42, 101888 (2021). This paper shows a comprehensive overview of the relation of protein glycosylation to redox regulation.
Chen, P. H., Chi, J. T. & Boyce, M. Functional crosstalk among oxidative stress and O-GlcNAc signaling pathways. Glycobiology 28, 556–564 (2018).
Tanaka, L. Y., Oliveira, P. V. S. & Laurindo, F. R. M. Peri/epicellular thiol oxidoreductases as mediators of extracellular redox signaling. Antioxid. Redox Signal. 33, 280–307 (2020). This work describes the role of extracellular thiol oxidoreductases in redox signalling.
Lorenzen, I., Eble, J. A. & Hanschmann, E. M. Thiol switches in membrane proteins — extracellular redox regulation in cell biology. Biol. Chem. 402, 253–269 (2021).
Palacio, P. L., Godoy, J. R., Aktas, O. & Hanschmann, E. M. Changing perspectives from oxidative stress to redox signaling-extracellular redox control in translational medicine. Antioxidants 11, 1181 (2022).
Forman, H. J., Bernardo, A. & Davies, K. J. What is the concentration of hydrogen peroxide in blood and plasma? Arch. Biochem. Biophys. 603, 48–53 (2016).
Hosogi, S. et al. Plasma membrane anchored nanosensor for quantifying endogenous production of H(2)O(2) in living cells. Biosens. Bioelectron. 179, 113077 (2021).
Go, Y. M. & Jones, D. P. The redox proteome. J. Biol. Chem. 288, 26512–26520 (2013). This paper provides an overview of the fundamentals of the redox proteome.
Hawkins, C. L. & Davies, M. J. Detection, identification, and quantification of oxidative protein modifications. J. Biol. Chem. 294, 19683–19708 (2019).
Cortese-Krott, M. M. et al. The reactive species interactome: evolutionary emergence, biological significance, and opportunities for redox metabolomics and personalized medicine. Antioxid. Redox Signal. 27, 684–712 (2017). This paper describes how reactive species interact among themselves and with their targets.
Malard, E., Valable, S., Bernaudin, M., Pérès, E. & Chatre, L. The reactive species interactome in the brain. Antioxid. Redox Signal. 35, 1176–1206 (2021).
Go, Y. M., Chandler, J. D. & Jones, D. P. The cysteine proteome. Free Radic. Biol. Med. 84, 227–245 (2015).
Reczek, C. R. & Chandel, N. S. ROS-dependent signal transduction. Curr. Opin. Cell Biol. 33, 8–13 (2015).
Cadenas-Garrido, P. et al. Using redox proteomics to gain new insights into neurodegenerative disease and protein modification. Antioxidants 13, 127 (2024).
Xiao, H. et al. A quantitative tissue-specific landscape of protein redox regulation during aging. Cell 180, 968–983 (2020). This work describes ‘Oximouse’, a quantitative data set of the mouse cysteine redox proteome in vivo.
Pham, T. K., Buczek, W. A., Mead, R. J., Shaw, P. J. & Collins, M. O. Proteomic approaches to study cysteine oxidation: applications in neurodegenerative diseases. Front. Mol. Neurosci. 14, 678837 (2021).
Fu, L. et al. Nucleophilic covalent ligand discovery for the cysteine redoxome. Nat. Chem. Biol. 19, 1309–1319 (2023).
Ferreira, R. B., Fu, L., Jung, Y., Yang, J. & Carroll, K. S. Reaction-based fluorogenic probes for detecting protein cysteine oxidation in living cells. Nat. Commun. 13, 5522 (2022).
Erdogan, Y. C. et al. Complexities of the chemogenetic toolkit: differential mDAAO activation by D-amino substrates and subcellular targeting. Free Radic. Biol. Med. 177, 132–142 (2021).
Steinhorn, B. et al. Chemogenetic generation of hydrogen peroxide in the heart induces severe cardiac dysfunction. Nat. Commun. 9, 4044 (2018).
Steinhorn, B., Eroglu, E. & Michel, T. Chemogenetic approaches to probe redox pathways: implications for cardiovascular pharmacology and toxicology. Annu. Rev. Pharmacol. Toxicol. 62, 551–571 (2021).
Nanadikar, M. S. et al. IDH3γ functions as a redox switch regulating mitochondrial energy metabolism and contractility in the heart. Nat. Commun. 14, 2123 (2023).
Kritsiligkou, P. et al. Proteome-wide tagging with an H(2)O(2) biosensor reveals highly localized and dynamic redox microenvironments. Proc. Natl Acad. Sci. USA 120, e2314043120 (2023). This work describes the complex nature of cellular redox microenvironments.
Feng, S. et al. Development of a clickable probe for profiling of protein glutathionylation in the central cellular metabolism of E. coli and Drosophila. Chem. Biol. 22, 1461–1469 (2015).
van der Reest, J., Lilla, S., Zheng, L., Zanivan, S. & Gottlieb, E. Proteome-wide analysis of cysteine oxidation reveals metabolic sensitivity to redox stress. Nat. Commun. 9, 1581 (2018).
Maret, W. The redox biology of redox-inert zinc ions. Free Radic. Biol. Med. 134, 311–326 (2019).
Hübner, C. & Haase, H. Interactions of zinc- and redox-signaling pathways. Redox Biol. 41, 101916 (2021).
Netto, L. E. S. & Machado, L. Preferential redox regulation of cysteine-based protein tyrosine phosphatases: structural and biochemical diversity. FEBS J. 289, 5480–5504 (2022).
Tossounian, M. A., Zhang, B. & Gout, I. The writers, readers, and erasers in redox regulation of GAPDH. Antioxidants 9, 1288 (2020).
Mailloux, R. J. Protein S-glutathionylation reactions as a global inhibitor of cell metabolism for the desensitization of hydrogen peroxide signals. Redox Biol. 32, 101472 (2020). This paper examines the role of protein S-glutathionylation in redox signalling.
Bodnar, Y. & Lillig, C. H. Cysteinyl and methionyl redox switches: structural prerequisites and consequences. Redox Biol. 65, 102832 (2023).
Reichmann, D. & Jakob, U. The roles of conditional disorder in redox proteins. Curr. Opin. Struct. Biol. 23, 436–442 (2013).
Devant, P. et al. Gasdermin D pore-forming activity is redox-sensitive. Cell Rep. 42, 112008 (2023).
Weismiller, H. A., Holub, T. J., Krzesinski, B. J. & Margittai, M. A thiol-based intramolecular redox switch in four-repeat tau controls fibril assembly and disassembly. J. Biol. Chem. 297, 101021 (2021).
Ulrich, K. et al. From guide to guard-activation mechanism of the stress-sensing chaperone Get3. Mol. Cell 82, 3226–3238.e7 (2022).
Moskovitz, J. & Smith, A. Methionine sulfoxide and the methionine sulfoxide reductase system as modulators of signal transduction pathways: a review. Amino Acids 53, 1011–1020 (2021).
Lim, J. M., Kim, G. & Levine, R. L. Methionine in proteins: it’s not just for protein initiation anymore. Neurochem. Res. 44, 247–257 (2019).
Kaya, A., Lee, B. C. & Gladyshev, V. N. Regulation of protein function by reversible methionine oxidation and the role of selenoprotein MsrB1. Antioxid. Redox Signal. 23, 814–822 (2015).
Labunskyy, V. M., Hatfield, D. L. & Gladyshev, V. N. Selenoproteins: molecular pathways and physiological roles. Physiol. Rev. 94, 739–777 (2014).
Zhang, Y. et al. Role of selenoproteins in redox regulation of signaling and the antioxidant system: a review. Antioxidants (Basel) 9, 383 (2020).
Nasim, M. J., Zuraik, M. M., Abdin, A. Y., Ney, Y. & Jacob, C. Selenomethionine: a pink trojan redox horse with implications in aging and various age-related diseases. Antioxidants 10, 882 (2021).
Assmann, A., Briviba, K. & Sies, H. Reduction of methionine selenoxide to selenomethionine by glutathione. Arch. Biochem. Biophys. 349, 201–203 (1998).
Giulivi, C., Traaseth, N. J. & Davies, K. J. Tyrosine oxidation products: analysis and biological relevance. Amino Acids 25, 227–232 (2003).
Zhou, X., Liu, F., Li, N. & Zhang, Y. Large-scale qualitative and quantitative assessment of dityrosine crosslinking omics in response to endogenous and exogenous hydrogen peroxide in Escherichia coli. Antioxidants 12, 786 (2023).
Piacenza, L., Zeida, A., Trujillo, M. & Radi, R. The superoxide radical switch in the biology of nitric oxide and peroxynitrite. Physiol. Rev. 102, 1881–1906 (2022). This paper is a comprehensive review on the role of reactive nitrogen species in redox regulation.
Griswold-Prenner, I. et al. Unveiling the human nitroproteome: protein tyrosine nitration in cell signaling and cancer. J. Biol. Chem. 299, 105038 (2023).
Cobley, J. N. Oxiforms: unique cysteine residue- and chemotype-specified chemical combinations can produce functionally-distinct proteoforms. Bioessays 45, e2200248 (2023).
Cobley, J. N. 50 Shades of oxidative stress: a state-specific cysteine redox pattern hypothesis. Redox Biol. 67, 102936 (2023).
Pak, V. V. et al. Ultrasensitive genetically encoded indicator for hydrogen peroxide identifies roles for the oxidant in cell migration and mitochondrial function. Cell Metab. 31, 642–653 (2020).
Morgan, B. et al. Real-time monitoring of basal H2O2 levels with peroxiredoxin-based probes. Nat. Chem. Biol. 12, 437–443 (2016).
Radzinski, M. et al. Temporal profiling of redox-dependent heterogeneity in single cells. eLife 7, e37623 (2018).
Braeckman, B. P., Smolders, A., Back, P. & De Henau, S. In vivo detection of reactive oxygen species and redox status in Caenorhabditis elegans. Antioxid. Redox Signal. 25, 577–592 (2016).
Knoefler, D. et al. Quantitative in vivo redox sensors uncover oxidative stress as an early event in life. Mol. Cell 47, 767–776 (2012).
Bazopoulou, D. et al. Developmental ROS individualizes organismal stress resistance and lifespan. Nature 576, 301–305 (2019).
Janke, R., Dodson, A. E. & Rine, J. Metabolism and epigenetics. Annu. Rev. Cell Dev. Biol. 31, 473–496 (2015).
Parkhitko, A. A., Filine, E., Mohr, S. E., Moskalev, A. & Perrimon, N. Targeting metabolic pathways for extension of lifespan and healthspan across multiple species. Ageing Res. Rev. 64, 101188 (2020).
Oleson, B. J. et al. Early life changes in histone landscape protect against age-associated amyloid toxicities through HSF-1-dependent regulation of lipid metabolism. Nat. Aging 4, 48–61 (2024). This paper examines redox-regulated histone methylation in relation to the regulation of lipid metabolism.
Dustin, C. M., Heppner, D. E., Lin, M. J. & van der Vliet, A. Redox regulation of tyrosine kinase signalling: more than meets the eye. J. Biochem. 167, 151–163 (2020).
Mitchell, S., Vargas, J. & Hoffmann, A. Signaling via the NFκB system. Wiley Interdiscip. Rev. Syst. Biol. Med. 8, 227–241 (2016).
Truong, T. H. et al. Molecular basis for redox activation of epidermal growth factor receptor kinase. Cell Chem. Biol. 23, 837–848 (2016).
Benhar, M. Oxidants, antioxidants and thiol redox switches in the control of regulated cell death pathways. Antioxidants 9, 309 (2020).
Korshavn, K. J., Wales, T. E., Bird, G. H., Engen, J. R. & Walensky, L. D. A redox switch regulates the structure and function of anti-apoptotic BFL-1. Nat. Struct. Mol. Biol. 27, 781–789 (2020).
Braunstein, I. et al. Opposing effects of polysulfides and thioredoxin on apoptosis through caspase persulfidation. J. Biol. Chem. 295, 3590–3600 (2020).
Jiang, X., Stockwell, B. R. & Conrad, M. Ferroptosis: mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 22, 266–282 (2021). This study shows a comprehensive overview of the role of ferroptosis in redox biology.
Seiler, A. et al. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab. 8, 237–248 (2008).
Llabani, E. et al. Diverse compounds from pleuromutilin lead to a thioredoxin inhibitor and inducer of ferroptosis. Nat. Chem. 11, 521–532 (2019).
Noguchi, T. et al. Nuclear-accumulated SQSTM1/p62-based ALIS act as microdomains sensing cellular stresses and triggering oxidative stress-induced parthanatos. Cell Death Dis. 9, 1193 (2018).
Scaturro, P. & Pichlmair, A. Oxeiptosis: a discreet way to respond to radicals. Curr. Opin. Immunol. 56, 37–43 (2019).
Pagliaro, P. & Penna, C. Inhibitors of NLRP3 inflammasome in ischemic heart disease: focus on functional and redox aspects. Antioxidants 12, 1396 (2023).
Peters, A., Nawrot, T. S. & Baccarelli, A. A. Hallmarks of environmental insults. Cell 184, 1455–1468 (2021).
Larigot, L. et al. Aryl hydrocarbon receptor and its diverse ligands and functions: an exposome receptor. Annu. Rev. Pharmacol. Toxicol. 62, 383–404 (2022).
Go, Y. M. & Jones, D. P. Redox biology: interface of the exposome with the proteome, epigenome and genome. Redox Biol. 2, 358–360 (2014).
Salminen, A. Aryl hydrocarbon receptor (AhR) reveals evidence of antagonistic pleiotropy in the regulation of the aging process. Cell Mol. Life Sci. 79, 489 (2022). This study is an overview of the pleiotropic role of aryl hydrocarbon receptor in biology, with particular reference to ageing.
Sondermann, N. C. et al. Functions of the aryl hydrocarbon receptor (AHR) beyond the canonical AHR/ARNT signaling pathway. Biochem. Pharmacol. 208, 115371 (2023).
Muri, J. & Kopf, M. Redox regulation of immunometabolism. Nat. Rev. Immunol. 21, 363–381 (2021). This review, together with that by Morris et al. (2022), summarizes the knowledge on redox regulation of the immune response.
Morris, G., Gevezova, M., Sarafian, V. & Maes, M. Redox regulation of the immune response. Cell Mol. Immunol. 19, 1079–1101 (2022). This review, together with that by Muri and Kopf (2021), summarizes the knowledge on redox regulation of the immune response.
Geng, S., Lin, R., Wu, Y., Wang, J. & Li, L. Modulation of innate immune memory dynamics by subcellular reactive oxygen species. Antioxid. Redox Signal. 39, 1027–1038 (2023).
Granados, J. C. et al. AHR is a master regulator of diverse pathways in endogenous metabolism. Sci. Rep. 12, 16625 (2022).
Rius-Pérez, S., Torres-Cuevas, I., Millán, I., Ortega, Á. L. & Pérez, S. PGC-1α, inflammation, and oxidative stress: an integrative view in metabolism. Oxid. Med. Cell Longev. 2020, 1452696 (2020).
Lui, P. Y., Jin, D. Y. & Stevenson, N. J. MicroRNA: master controllers of intracellular signaling pathways. Cell Mol. Life Sci. 72, 3531–3542 (2015).
Buler, M., Andersson, U. & Hakkola, J. Who watches the watchmen? Regulation of the expression and activity of sirtuins. FASEB J. 30, 3942–3960 (2016).
Putker, M. & O’Neill, J. S. Reciprocal control of the circadian clock and cellular redox state — a critical appraisal. Mol. Cell 39, 6–19 (2016).
Di Mascio, P. et al. Singlet molecular oxygen reactions with nucleic acids, lipids, and proteins. Chem. Rev. 119, 2043–2086 (2019).
Brash, D. E. & Goncalves, L. C. P. Chemiexcitation: mammalian photochemistry in the dark†. Photochem. Photobiol. 99, 251–276 (2023).
Spickett, C. M. Formation of oxidatively modified lipids as the basis for a cellular epilipidome. Front. Endocrinol. 11, 602771 (2020).
Doka, E. et al. Control of protein function through oxidation and reduction of persulfidated states. Sci. Adv. 6, eaax8358 (2020).
Lopez-Otin, C. & Kroemer, G. Hallmarks of health. Cell 184, 33–63 (2021).
Ayres, J. S. The biology of physiological health. Cell 181, 250–269 (2020).
Lopez-Otin, C., Pietrocola, F., Roiz-Valle, D., Galluzzi, L. & Kroemer, G. Meta-hallmarks of aging and cancer. Cell Metab. 35, 12–35 (2023).
Feelisch, M., Cortese-Krott, M. M., Santolini, J., Wootton, S. A. & Jackson, A. A. Systems redox biology in health and disease. EXCLI J. 21, 623–646 (2022).
Heusch, G. et al. Health position paper and redox perspectives on reactive oxygen species as signals and targets of cardioprotection. Redox Biol. 67, 102894 (2023).
Sörensen, M. et al. Health position paper and redox perspectives — disease burden by transportation noise. Redox Biol. 69, 102995 (2023).
Meng, J. et al. Precision redox: the key for antioxidant pharmacology. Antioxid. Redox Signal. 34, 1069–1082 (2021). This paper describes the challenges for the future of precision redox-based therapy.
Nogales, C. et al. Network pharmacology: curing causal mechanisms instead of treating symptoms. Trends Pharmacol. Sci. 43, 136–150 (2022).
Wang, R. S., Maron, B. A. & Loscalzo, J. Multiomics network medicine approaches to precision medicine and therapeutics in cardiovascular diseases. Arterioscler. Thromb. Vasc. Biol. 43, 493–503 (2023).
Halliwell, B. Understanding mechanisms of antioxidant action in health and disease. Nat. Rev. Mol. Cell Biol. 25, 13–33 (2024). This study is a comprehensive overview of mechanisms of antioxidant action.
Hayes, J. D., Dinkova-Kostova, A. T. & Tew, K. D. Oxidative stress in cancer. Cancer Cell 38, 167–197 (2020).
Zhang, J. et al. Systematic identification of anticancer drug targets reveals a nucleus-to-mitochondria ROS-sensing pathway. Cell 186, 2361–2379.e25 (2023).
Krafczyk, N. & Klotz, L. O. FOXO transcription factors in antioxidant defense. IUBMB Life 74, 53–61 (2022).
Rodriguez-Colman, M. J., Dansen, T. B. & Burgering, B. M. T. FOXO transcription factors as mediators of stress adaptation. Nat. Rev. Mol. Cell Biol. 25, 46–64 (2024).
Lee, P., Chandel, N. S. & Simon, M. C. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat. Rev. Mol. Cell Biol. 21, 268–283 (2020).
Liu, X., Xu, C., Xiao, W. & Yan, N. Unravelling the role of NFE2L1 in stress responses and related diseases. Redox Biol. 65, 102819 (2023).
Katsuoka, F., Otsuki, A., Hatanaka, N., Okuyama, H. & Yamamoto, M. Target gene diversity of the Nrf1-MafG transcription factor revealed by a tethered heterodimer. Mol. Cell Biol. 42, e0052021 (2022).
Hu, S. et al. Nrf1 is an indispensable redox-determining factor for mitochondrial homeostasis by integrating multi-hierarchical regulatory networks. Redox Biol. 57, 102470 (2022).
Suzuki, T., Takahashi, J. & Yamamoto, M. Molecular basis of the KEAP1-NRF2 signaling pathway. Mol. Cell 46, 133–141 (2023).
Ibrahim, L. et al. Defining the functional targets of cap‘n’collar transcription factors NRF1, NRF2, and NRF3. Antioxidants 9, 1025 (2020).
Choi, H. I. et al. PGC-1alpha attenuates hydrogen peroxide-induced apoptotic cell death by upregulating Nrf-2 via GSK3beta inactivation mediated by activated p38 in HK-2 cells. Sci. Rep. 7, 4319 (2017).
Shi, T., van Soest, D. M. K., Polderman, P. E., Burgering, B. M. T. & Dansen, T. B. DNA damage and oxidant stress activate p53 through differential upstream signaling pathways. Free Radic. Biol. Med. 172, 298–311 (2021).
Rothhammer, V. & Quintana, F. J. The aryl hydrocarbon receptor: an environmental sensor integrating immune responses in health and disease. Nat. Rev. Immunol. 19, 184–197 (2019).
Scholtes, C. & Giguère, V. Transcriptional regulation of ROS homeostasis by the ERR subfamily of nuclear receptors. Antioxidants 10, 437 (2021).
Truong, T. H. & Carroll, K. S. Redox regulation of epidermal growth factor receptor signaling through cysteine oxidation. Biochemistry 51, 9954–9965 (2012).
Pastore, A. & Piemonte, F. S-glutathionylation signaling in cell biology: progress and prospects. Eur. J. Pharm. Sci. 46, 279–292 (2012).
Carter, E. L. & Ragsdale, S. W. Modulation of nuclear receptor function by cellular redox poise. J. Inorg. Biochem. 133, 92–103 (2014).
Shao, D. et al. A redox-dependent mechanism for regulation of AMPK activation by thioredoxin1 during energy starvation. Cell Metab. 19, 232–245 (2014).
Zhao, Y. et al. ROS signaling under metabolic stress: cross-talk between AMPK and AKT pathway. Mol. Cancer 16, 79 (2017).
Xiong, Y., Uys, J. D., Tew, K. D. & Townsend, D. M. S-glutathionylation: from molecular mechanisms to health outcomes. Antioxid. Redox Signal. 15, 233–270 (2011).
Singh, C. K. et al. The role of sirtuins in antioxidant and redox signaling. Antioxid. Redox Signal. 28, 643–661 (2018).
Kalous, K. S., Wynia-Smith, S. L. & Smith, B. C. Sirtuin oxidative post-translational modifications. Front. Physiol. 12, 763417 (2021).
Heppner, D. E. Structural insights into redox-active cysteine residues of the Src family kinases. Redox Biol. 41, 101934 (2021).
Wu, Y., Lim, Y. W. & Parton, R. G. Caveolae and the oxidative stress response. Biochem. Soc. Trans. 51, 1377–1385 (2023).
Monico, A., Guzman-Caldentey, J., Pajares, M. A., Martin-Santamaria, S. & Perez-Sala, D. Molecular insight into the regulation of vimentin by cysteine modifications and zinc binding. Antioxidants 10, 1039 (2021).
Zhang, Y. et al. RIP1 autophosphorylation is promoted by mitochondrial ROS and is essential for RIP3 recruitment into necrosome. Nat. Commun. 8, 14329 (2017).
Mittler, R. & Jones, D. P. The redox code of plants. Plant Cell Environ. https://doi.org/10.1111/pce.14787 (2023).
Oka, S. I., Titus, A. S., Zablocki, D. & Sadoshima, J. Molecular properties and regulation of NAD(+) kinase (NADK). Redox Biol. 59, 102561 (2023).
Kampjut, D. & Sazanov, L. A. Structure and mechanism of mitochondrial proton-translocating transhydrogenase. Nature 573, 291–295 (2019).
Francisco, A., Figueira, T. R. & Castilho, R. F. Mitochondrial NAD(P)(+) transhydrogenase: from molecular features to physiology and disease. Antioxid. Redox Signal. 36, 864–884 (2022).
Katsyuba, E., Romani, M., Hofer, D. & Auwerx, J. NAD(+) homeostasis in health and disease. Nat. Metab. 2, 9–31 (2020).
Netto, L. E. & Antunes, F. The roles of peroxiredoxin and thioredoxin in hydrogen peroxide sensing and in signal transduction. Mol. Cell 39, 65–71 (2016).
Peskin, A. V. et al. The high reactivity of peroxiredoxin 2 with H(2)O(2) is not reflected in its reaction with other oxidants and thiol reagents. J. Biol. Chem. 282, 11885–11892 (2007).
Sobotta, M. C. et al. Peroxiredoxin-2 and STAT3 form a redox relay for H2O2 signaling. Nat. Chem. Biol. 11, 64–70 (2015). This study shows a prototypical example of redox relay in hydrogen peroxide signalling.
Garrido Ruiz, D., Sandoval-Perez, A., Rangarajan, A. V., Gunderson, E. L. & Jacobson, M. P. Cysteine oxidation in proteins: structure, biophysics, and simulation. Biochemistry 61, 2165–2176 (2022).
Chen, S. M. & Tang, X. Q. Homocysteinylation and sulfhydration in diseases. Curr. Neuropharmacol. 20, 1726–1735 (2022).
Gout, I. Coenzyme A, protein CoAlation and redox regulation in mammalian cells. Biochem. Soc. Trans. 46, 721–728 (2018).
Nakamura, T., Oh, C. K., Zhang, X., Tannenbaum, S. R. & Lipton, S. A. Protein transnitrosylation signaling networks contribute to inflammaging and neurodegenerative disorders. Antioxid. Redox Signal. 35, 531–550 (2021).
Mustafa, A. K. et al. H2S signals through protein S-sulfhydration. Sci. Signal. 2, ra72 (2009).
Wensien, M. et al. A lysine–cysteine redox switch with an NOS bridge regulates enzyme function. Nature 593, 460–464 (2021).
Rabe von Pappenheim, F. et al. Widespread occurrence of covalent lysine–cysteine redox switches in proteins. Nat. Chem. Biol. 18, 368–375 (2022).
Dennis, K. K., Go, Y. M. & Jones, D. P. Redox systems biology of nutrition and oxidative stress. J. Nutr. 149, 553–565 (2019).
Vogel, C. F. A., Van Winkle, L. S., Esser, C. & Haarmann-Stemmann, T. The aryl hydrocarbon receptor as a target of environmental stressors — implications for pollution mediated stress and inflammatory responses. Redox Biol. 34, 101530 (2020).
Gargaro, M. et al. The landscape of AhR regulators and coregulators to fine-tune AhR functions. Int. J. Mol. Sci. 22, 757 (2021).
Vogeley, C. et al. Unraveling the differential impact of PAHs and dioxin-like compounds on AKR1C3 reveals the EGFR extracellular domain as a critical determinant of the AHR response. Environ. Int. 158, 106989 (2022).
Hu, X. et al. A scalable workflow to characterize the human exposome. Nat. Commun. 12, 5575 (2021).
Frenis, K. et al. Redox switches in noise-induced cardiovascular and neuronal dysregulation. Front. Mol. Biosci. 8, 784910 (2021).
Münzel, T., Sorensen, M., Hahad, O., Nieuwenhuijsen, M. & Daiber, A. The contribution of the exposome to the burden of cardiovascular disease. Nat. Rev. Cardiol. 20, 651–669 (2023).
Krutmann, J., Schikowski, T., Morita, A. & Berneburg, M. Environmentally-induced (extrinsic) skin aging: exposomal factors and underlying mechanisms. J. Invest. Dermatol. 141, 1096–1103 (2021).
Ghezzi, P. & Rubartelli, A. Redox regulation of defense against bacterial and viral pathogens. Curr. Opin. Chem. Biol. 76, 102339 (2023).
Dumas, A. & Knaus, U. G. Raising the ‘good’ oxidants for immune protection. Front. Immunol. 12, 698042 (2021).
Kunst, C. et al. The influence of gut microbiota on oxidative stress and the immune system. Biomedicines 11, 1388 (2023).
Cobley, J. N. How exercise induces oxidative eustress. in Oxidative Stress: Eustress and Distress (ed. Sies, H.) 447–462 (Academic Press, 2020).
Margaritelis, N. V., Paschalis, V., Theodorou, A. A., Kyparos, A. & Nikolaidis, M. G. Redox basis of exercise physiology. Redox Biol. 35, 101499 (2020).
Lisi, V. et al. Steady-state redox status in circulating extracellular vesicles: a proof-of-principle study on the role of fitness level and short-term aerobic training in healthy young males. Free Radic. Biol. Med. 204, 266–275 (2023).
Felix-Soriano, E. & Stanford, K. I. Exerkines and redox homeostasis. Redox Biol. 63, 102748 (2023).
Radak, Z. et al. Epigenetic and ‘redoxogenetic’ adaptation to physical exercise. Free Radic. Biol. Med. 210, 65–74 (2024).
Acknowledgements
The authors are grateful to D. P. Jones, a longtime friend and colleague, for his substantial input and advice. B. Oleson helped with preparing figures. The authors also thank many researchers active in redox biology, who have generated an impressive literature that could not be covered exhaustively in the present overview. H.S. acknowledges longstanding support by Deutsche Forschungsgemeinschaft, Bonn, Germany, and by the National Foundation for Cancer Research, Bethesda, MD, USA. R.J.M. acknowledges Natural Sciences and Engineering Research Council of Canada Discovery Grant Program (RGPIN-2022-03240). U.J. acknowledges the National Institute of Health grant R35 GM122506 and the National Institute of Aging grant R21 AG078540.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the Review.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Molecular Cell Biology thanks Tobias Dansen, who co-reviewed with Janneke Keijer, Peter Nagy and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
‘Oximouse’: https://oximouse.hms.harvard.edu
Glossary
- Antioxidants
-
Any substance that prevents, delays or removes oxidative damage. In biology, this includes various antioxidant enzymes and low-molecular-weight antioxidant compounds.
- Hormesis
-
This term describes a biphasic dose–response phenomenon, whereby low-dose exposure leads to stimulation or preconditioning and high-dose exposure causes inhibition. The phenomenon is often described by a J-shaped or inverted U-shaped dose–response curve.
- Oxidative distress
-
Biomolecular damage upon supraphysiological exposure to oxidants, resulting in detrimental consequence to life processes.
- p53 pathway
-
A tumour suppression pathway, which oversees cell cycle regulation, DNA repair and cell death mechanisms to halt the proliferation of aberrant cells.
- Redox stress signalling threshold
-
(RST). Condition at which the oxidant or reductant load causes the transition from beneficial (eustress) to detrimental (distress) outcome.
- Surface-enhanced Raman spectroscopy
-
(SERS). A surface-sensitive method that amplifies Raman scattering from molecules absorbed on metal surfaces or nanostructures.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sies, H., Mailloux, R.J. & Jakob, U. Fundamentals of redox regulation in biology. Nat Rev Mol Cell Biol (2024). https://doi.org/10.1038/s41580-024-00730-2
Accepted:
Published:
DOI: https://doi.org/10.1038/s41580-024-00730-2