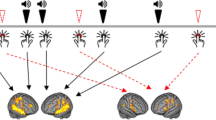
Abstract
Perceptual disturbances in psychosis, such as auditory verbal hallucinations, are associated with increased baseline activity in the associative auditory cortex and increased dopamine transmission in the associative striatum. Perceptual disturbances are also associated with perceptual biases that suggest increased reliance on prior expectations. We review theoretical models of perceptual inference and key supporting physiological evidence, as well as the anatomy of associative cortico–striatal loops that may be relevant to auditory perceptual inference. Integrating recent findings, we outline a working framework that bridges neurobiology and the phenomenology of perceptual disturbances via theoretical models of perceptual inference.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
10 March 2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Fletcher, P. C. & Frith, C. D. Perceiving is believing: a Bayesian approach to explaining the positive symptoms of schizophrenia. Nat. Rev. Neurosci. 10, 48–58 (2009). This in-depth review proposes an influential framework based on Bayesian inference to bridge the known dopamine dysregulation and individual experiential aspects associated with psychosis.
Weinstein, J. J. et al. Pathway-specific dopamine abnormalities in schizophrenia. Biol. Psychiatry 81, 31–42 (2017). This study reviews the literature on dopamine alterations in schizophrenia with special emphasis on the distinct anatomical pathways that comprise the dopamine system.
Powers, A. R. III, Kelley, M. & Corlett, P. R. Hallucinations as top-down effects on perception. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 1, 393–400 (2016).
Adams, R. A., Stephan, K. E., Brown, H. R., Frith, C. D. & Friston, K. J. The computational anatomy of psychosis. Front. Psychiatry 4, 47 (2013). This report provides a comprehensive computational account of various phenomenological and neurophysiological aspects of schizophrenia.
Sterzer, P. et al. The predictive coding account of psychosis. Biol. Psychiatry 84, 634–643 (2018).
Heinz, A. Dopaminergic dysfunction in alcoholism and schizophrenia—psychopathological and behavioral correlates. Eur. Psychiatry 17, 9–16 (2002).
Kapur, S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am. J. Psychiatry 160, 13–23 (2003).
Glimcher, P. W. Understanding dopamine and reinforcement learning: the dopamine reward prediction error hypothesis. Proc. Natl Acad. Sci. USA 108 (Suppl. 3), 15647–15654 (2011).
Schultz, W. Predictive reward signal of dopamine neurons. J. Neurophysiol. 80, 1–27 (1998).
Schultz, W. Getting formal with dopamine and reward. Neuron 36, 241–263 (2002).
Schultz, W. Multiple dopamine functions at different time courses. Annu. Rev. Neurosci. 30, 259–288 (2007).
Yung, A. R. & McGorry, P. D. The prodromal phase of first-episode psychosis: past and current conceptualizations. Schizophr. Bull. 22, 353–370 (1996).
Lim, A., Hoek, H. W., Deen, M. L., Blom, J. D. & GROUP Investigators. Prevalence and classification of hallucinations in multiple sensory modalities in schizophrenia spectrum disorders. Schizophr. Res. 176, 493–499 (2016).
Waters, F. & Fernyhough, C. Hallucinations: a systematic review of points of similarity and difference across diagnostic classes. Schizophr. Bull. 43, 32–43 (2017).
Andreasen, N. C. & Flaum, M. Schizophrenia: the characteristic symptoms. Schizophr. Bull. 17, 27–49 (1991).
Nayani, T. H. & David, A. S. The auditory hallucination: a phenomenological survey. Psychol. Med. 26, 177–189 (1996).
Llorca, P. M. et al. Hallucinations in schizophrenia and Parkinson’s disease: an analysis of sensory modalities involved and the repercussion on patients. Sci. Rep. 6, 38152 (2016).
Lehembre-Shiah, E. et al. Distinct relationships between visual and auditory perceptual abnormalities and conversion to psychosis in a clinical high-risk population. JAMA Psychiatry 74, 104–106 (2017).
Emsley, R., Rabinowitz, J., Torreman, M. & RIS-INT-35 Early Psychosis Global Working Group. The factor structure for the Positive and Negative Syndrome Scale (PANSS) in recent-onset psychosis. Schizophr. Res. 61, 47–57 (2003).
Freedman, R. Schizophrenia. N. Engl. J. Med. 349, 1738–1749 (2003).
De Keyser, J., De Backer, J. P., Ebinger, G. & Vauquelin, G. Regional distribution of the dopamine D2 receptors in the mesotelencephalic dopamine neuron system of human brain. J. Neurol. Sci. 71, 119–127 (1985).
Palacios, J. M., Camps, M., Cortes, R. & Probst, A. Mapping dopamine receptors in the human brain. J. Neural. Transm. Suppl. 27, 227–235 (1988).
Agid, O., Seeman, P. & Kapur, S. The ‘delayed onset’ of antipsychotic action—an idea whose time has come and gone. J. Psychiatry Neurosci. 31, 93–100 (2006).
Emsley, R., Rabinowitz, J. & Medori, R. Time course for antipsychotic treatment response in first-episode schizophrenia. Am. J. Psychiatry 163, 743–745 (2006).
Rector, N. A. & Beck, A. T. Cognitive behavioral therapy for schizophrenia: an empirical review. J. Nerv. Ment. Dis. 189, 278–287 (2001).
Slotema, C. W., Aleman, A., Daskalakis, Z. J. & Sommer, I. E. Meta-analysis of repetitive transcranial magnetic stimulation in the treatment of auditory verbal hallucinations: update and effects after one month. Schizophr. Res. 142, 40–45 (2012).
Pruessner, J. C., Champagne, F., Meaney, M. J. & Dagher, A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C]raclopride. J. Neurosci. 24, 2825–2831 (2004).
Volkow, N. D., Fowler, J. S., Wang, G. J. & Swanson, J. M. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol. Psychiatry 9, 557–569 (2004).
Jardri, R. et al. Are hallucinations due to an imbalance between excitatory and inhibitory influences on the brain? Schizophr. Bull. 42, 1124–1134 (2016).
Krystal, J. H. et al. impaired tuning of neural ensembles and the pathophysiology of schizophrenia: a translational and computational neuroscience perspective. Biol. Psychiatry 81, 874–885 (2017).
Bohlken, M. M., Hugdahl, K. & Sommer, I. E. Auditory verbal hallucinations: neuroimaging and treatment. Psychol. Med. 47, 199–208 (2017).
Goghari, V. M., Harrow, M., Grossman, L. S. & Rosen, C. A 20-year multi-follow-up of hallucinations in schizophrenia, other psychotic, and mood disorders. Psychol. Med. 43, 1151–1160 (2013).
Marshall, M. et al. Association between duration of untreated psychosis and outcome in cohorts of first-episode patients: a systematic review. Arch. Gen. Psychiatry 62, 975–983 (2005).
Summerfield, C. & de Lange, F. P. Expectation in perceptual decision making: neural and computational mechanisms. Nat. Rev. Neurosci. 15, 745–756 (2014). This review integrates non-primate electrophysiology and human neuroimaging findings that represent possible neural implementations of expectation effects in perceptual decision-making in the brain.
Bitzer, S., Park, H., Blankenburg, F. & Kiebel, S. J. Perceptual decision making: drift-diffusion model is equivalent to a Bayesian model. Front. Hum. Neurosci. 8, 102 (2014).
Friston, K. & Kiebel, S. Predictive coding under the free-energy principle. Philos Trans. R. Soc. Lond. B. Biol. Sci. 364, 1211–1221 (2009).
Rao, R. P. & Ballard, D. H. Predictive coding in the visual cortex: a functional interpretation of some extra-classical receptive-field effects. Nat. Neurosci. 2, 79–87 (1999).
Friston, K. Hallucinations and perceptual inference. Behavioral and Brain Sciences 28, 764–766 (2005). In this short commentary, Karl Friston delineates for the first time a perceptual-inference model of hallucinations.
Benrimoh, D., Parr, T., Vincent, P., Adams, R. A. & Friston, K. Active inference and auditory hallucinations. Comput. Psychiatr. 2, 183–204 (2018).
Bentall, R. P. & Slade, P. D. Reality testing and auditory hallucinations: a signal detection analysis. Br. J. Clin. Psychol. 24 (Pt 3), 159–169 (1985).
Brookwell, M. L., Bentall, R. P. & Varese, F. Externalizing biases and hallucinations in source-monitoring, self-monitoring and signal detection studies: a meta-analytic review. Psychol. Med. 43, 2465–2475 (2013).
Varese, F., Barkus, E. & Bentall, R. P. Dissociation mediates the relationship between childhood trauma and hallucination-proneness. Psychol. Med. 42, 1025–1036 (2012).
Vercammen, A., de Haan, E. H. & Aleman, A. Hearing a voice in the noise: auditory hallucinations and speech perception. Psychol. Med. 38, 1177–1184 (2008).
Powers, A. R., Mathys, C. & Corlett, P. R. Pavlovian conditioning-induced hallucinations result from overweighting of perceptual priors. Science 357, 596–600 (2017).
O’Callaghan, C. et al. Visual hallucinations are characterized by impaired sensory evidence accumulation: insights from hierarchical drift diffusion modeling in Parkinson’s Disease. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2, 680–688 (2017).
Limongi, R., Bohaterewicz, B., Nowicka, M., Plewka, A. & Friston, K. J. Knowing when to stop: aberrant precision and evidence accumulation in schizophrenia. Schizophr. Res. 197, 386–391 (2018).
Teufel, C. et al. Shift toward prior knowledge confers a perceptual advantage in early psychosis and psychosis-prone healthy individuals. Proc. Natl Acad. Sci. USA 112, 13401–13406 (2015). This behavioural human study provided a first demonstration of exaggerated expectation biases specifically relevant to perceptual disturbances in populations at risk for psychosis.
Cassidy, C. M. et al. A perceptual inference mechanism for hallucinations linked to striatal dopamine. Curr. Biol. 28, 503–514 (2018). This study is the first demonstration of a correlation between striatal dopamine excess and altered expectation biases in relation to hallucinations in patients with schizophrenia.
Gold, J. I. & Shadlen, M. N. The neural basis of decision making. Annu. Rev. Neurosci. 30, 535–574 (2007). This general review delves into the electrophysiology literature on perceptual decision making and its links to computational models of evidence accumulation.
Dehaene, S. & Changeux, J. P. Experimental and theoretical approaches to conscious processing. Neuron 70, 200–227 (2011).
Maia, T. V. & Cleeremans, A. Consciousness: converging insights from connectionist modeling and neuroscience. Trends Cogn. Sci. 9, 397–404 (2005).
de Lafuente, V. & Romo, R. Neuronal correlates of subjective sensory experience. Nat. Neurosci. 8, 1698–1703 (2005).
de Lafuente, V. & Romo, R. Neural correlate of subjective sensory experience gradually builds up across cortical areas. Proc. Natl Acad. Sci. USA 103, 14266–14271 (2006).
Van Vugt, B. et al. The threshold for conscious report: signal loss and response bias in visual and frontal cortex. Science 360, 537–542 (2018).
Kaas, J. H. & Hackett, T. A. Subdivisions of auditory cortex and processing streams in primates. Proc. Natl Acad. Sci. USA 97, 11793–11799 (2000).
Tang, C., Hamilton, L. S. & Chang, E. F. Intonational speech prosody encoding in the human auditory cortex. Science 357, 797–801 (2017).
Penfield, W. & Perot, P. The brain’s record of auditory and visual experience: a final summary and discussion. Brain 86, 595–696 (1963).
Kobayashi, E. et al. Magnetic resonance imaging abnormalities in familial temporal lobe epilepsy with auditory auras. Arch. Neurol. 60, 1546–1551 (2003).
Winawer, M. R., Ottman, R., Hauser, W. A. & Pedley, T. A. Autosomal dominant partial epilepsy with auditory features: defining the phenotype. Neurology 54, 2173–2176 (2000).
Yeterian, E. H. & Pandya, D. N. Corticostriatal connections of the superior temporal region in rhesus monkeys. J. Comp. Neurol. 399, 384–402 (1998). This tracing study in non-human primates describes the anatomical downstream connections of the superior temporal cortex.
Hackett, T. A., Stepniewska, I. & Kaas, J. H. Thalamocortical connections of the parabelt auditory cortex in macaque monkeys. J. Comp. Neurol. 400, 271–286 (1998).
Middleton, F. A. & Strick, P. L. The temporal lobe is a target of output from the basal ganglia. Proc. Natl Acad. Sci. USA 93, 8683–8687 (1996).
Shammah-Lagnado, S. J., Alheid, G. F. & Heimer, L. Efferent connections of the caudal part of the globus pallidus in the rat. J. Comp. Neurol. 376, 489–507 (1996).
Horga, G., Schatz, K. C., Abi-Dargham, A. & Peterson, B. S. Deficits in predictive coding underlie hallucinations in schizophrenia. J. Neurosci. 34, 8072–8082 (2014).
Jardri, R., Pouchet, A., Pins, D. & Thomas, P. Cortical activations during auditory verbal hallucinations in schizophrenia: a coordinate-based meta-analysis. Am. J. Psychiatry 168, 73–81 (2011).
Diederen, K. M. et al. Deactivation of the parahippocampal gyrus preceding auditory hallucinations in schizophrenia. Am. J. Psychiatry 167, 427–435 (2010).
Hoffman, R. E., Pittman, B., Constable, R. T., Bhagwagar, Z. & Hampson, M. Time course of regional brain activity accompanying auditory verbal hallucinations in schizophrenia. Br. J. Psychiatry 198, 277–283 (2011).
Gerfen, C. R. & Surmeier, D. J. Modulation of striatal projection systems by dopamine. Annu. Rev. Neurosci. 34, 441–466 (2011).
Maia, T. V. & Frank, M. J. An integrative perspective on the role of dopamine in schizophrenia. Biol. Psychiatry 81, 52–66 (2017). This article provides a comprehensive model of how dopamine dysregulation in schizophrenia may explain different symptom domains via reinforcement-learning theory.
Alexander, G. E., DeLong, M. R. & Strick, P. L. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 9, 357–381 (1986).
Haber, S. N. The primate basal ganglia: parallel and integrative networks. J. Chem. Neuroanat. 26, 317–330 (2003).
Choi, E. Y., Tanimura, Y., Vage, P. R., Yates, E. H. & Haber, S. N. Convergence of prefrontal and parietal anatomical projections in a connectional hub in the striatum. Neuroimage 146, 821–832 (2017).
Wickens, J. R., Reynolds, J. N. & Hyland, B. I. Neural mechanisms of reward-related motor learning. Curr. Opin. Neurobiol. 13, 685–690 (2003).
Xiong, Q., Znamenskiy, P. & Zador, A. M. Selective corticostriatal plasticity during acquisition of an auditory discrimination task. Nature 521, 348–351 (2015).
Redgrave, P., Prescott, T. J. & Gurney, K. Is the short-latency dopamine response too short to signal reward error? Trends Neurosci. 22, 146–151 (1999).
Horvitz, J. C., Stewart, T. & Jacobs, B. L. Burst activity of ventral tegmental dopamine neurons is elicited by sensory stimuli in the awake cat. Brain Res. 759, 251–258 (1997).
de Lafuente, V. & Romo, R. Dopamine neurons code subjective sensory experience and uncertainty of perceptual decisions. Proc. Natl Acad. Sci. USA 108, 19767–19771 (2011). This non-human primate study shows that dopamine neurons encode perceptual uncertainty during a decision-making task.
Lak, A., Nomoto, K., Keramati, M., Sakagami, M. & Kepecs, A. Midbrain dopamine neurons signal belief in choice accuracy during a perceptual decision. Curr. Biol. 27, 821–832 (2017).
Sarno, S., de Lafuente, V., Romo, R. & Parga, N. Dopamine reward prediction error signal codes the temporal evaluation of a perceptual decision report. Proc. Natl Acad. Sci. USA 114, E10494–E10503 (2017).
Sharpe, M. J. et al. Dopamine transients are sufficient and necessary for acquisition of model-based associations. Nat. Neurosci. 20, 735–742 (2017). This rodent study uses a sensory preconditioning paradigm and optogenetics to show that dopamine is necessary for stimulus–stimulus learning, separate from reward learning.
Menegas, W., Babayan, B. M., Uchida, N. & Watabe-Uchida, M. Opposite initialization to novel cues in dopamine signaling in ventral and posterior striatum in mice. eLife 6, e21886 (2017). This rodent study shows that, in contrast with dopamine signals in ventral striatum, those in dorsal striatum encode a type of sensory prediction error.
Wolpe, N. et al. Sensory attenuation in Parkinson’s disease is related to disease severity and dopamine dose. Sci. Rep. 8, 15643 (2018).
Vilares, I. & Kording, K. P. Dopaminergic medication increases reliance on current information in Parkinson’s Disease. Nat. Hum. Behav. 1, 0129 (2017).
Nour, M. M. et al. Dopaminergic basis for signaling belief updates, but not surprise, and the link to paranoia. Proc. Natl Acad. Sci. USA 115, E10167–E10176 (2018).
Howes, O. D. et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch. Gen. Psychiatry 69, 776–786 (2012).
Laruelle, M. Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J. Cereb. Blood Flow Metab. 20, 423–451 (2000).
Abi-Dargham, A. et al. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J. Neurosci. 22, 3708–3719 (2002).
Abi-Dargham, A. et al. Increased prefrontal cortical D1 receptors in drug naive patients with schizophrenia: a PET study with [11C]NNC112. J. Psychopharmacol. 26, 794–805 (2012).
Okubo, Y. et al. Decreased prefrontal dopamine D1 receptors in schizophrenia revealed by PET. Nature 385, 634–636 (1997).
Okubo, Y., Suhara, T., Sudo, Y. & Toru, M. Possible role of dopamine D1 receptors in schizophrenia. Mol. Psychiatr. 2, 291–292 (1997).
Laruelle, M. et al. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug free schizophrenic subjects. Proc. Natl Acad. Sci. USA 93, 9235–9240 (1996).
Abi-Dargham, A. et al. Increased striatal dopamine transmission in schizophrenia: confirmation in a second cohort. Am. J. Psychiatry 155, 761–767 (1998).
Abi-Dargham, A. et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc. Natl Acad. Sci. USA 97, 8104–8109 (2000).
Kegeles, L. et al. Increased synaptic dopamine in associative regions of the striatum in schizophrenia. Archives of General Psychiatry 67, 231–239 (2010).
Howes, O. D. et al. Dopamine synthesis capacity before onset of psychosis: a prospective [18f]-dopa pet imaging study. Am. J. Psychiatry 168, 1311–1317 (2011).
Howes, O. et al. Progressive increase in striatal dopamine synthesis capacity as patients develop psychosis: a PET study. Mol Psychiatry 16, 885–886 (2011).
Howes, O. D. et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch. Gen. Psychiatry 66, 13–20 (2009).
Laruelle, M., Abi-Dargham, A., Gil, R., Kegeles, L. & Innis, R. Increased dopamine transmission in schizophrenia: relationship to illness phases. Biol. Psychiatry 46, 56–72 (1999).
Thompson, J. L. et al. Striatal dopamine release in schizophrenia comorbid with substance dependence. Mol. Psychiatry 18, 909–915 (2013).
Jauhar, S. et al. A test of the transdiagnostic dopamine hypothesis of psychosis using positron emission tomographic imaging in bipolar affective disorder and schizophrenia. JAMA Psychiatry 74, 1206–1213 (2017).
Farde, L., Hall, H., Ehrin, E. & Sedvall, G. Quantitative analysis of D2 dopamine receptor binding in the living human brain by PET. Science 231, 258–261 (1986).
Farde, L. et al. Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine: relation to extrapyramidal side effects. Arch. Gen. Psychiatry 49, 538–544 (1992).
Farid, F. & Mahadun, P. Schizophrenia-like psychosis following left putamen infarct: a case report. J. Med. Case Rep. 3, 7337 (2009).
Kitabayashi, Y. et al. Schizophrenia-like psychosis following right putaminal infarction. J. Neuropsychiatry Clin. Neurosci. 18, 561–562 (2006).
Cleghorn, J. M. et al. Toward a brain map of auditory hallucinations. Am. J. Psychiatry 149, 1062–1069 (1992).
Horga, G. et al. Differential brain glucose metabolic patterns in antipsychotic-naive first-episode schizophrenia with and without auditory verbal hallucinations. J. Psychiatry Neurosci. 36, 312–321 (2011).
Zhuo, C. et al. Cerebral blood flow alterations specific to auditory verbal hallucinations in schizophrenia. Br. J. Psychiatry 210, 209–215 (2017).
Samejima, K. & Doya, K. Multiple representations of belief states and action values in corticobasal ganglia loops. Ann. N. Y. Acad. Sci. 1104, 213–228 (2007).
Ding, L. & Gold, J. I. Caudate encodes multiple computations for perceptual decisions. J. Neurosci. 30, 15747–15759 (2010).
Yartsev, M. M., Hanks, T. D., Yoon, A. M. & Brody, C. D. Causal contribution and dynamical encoding in the striatum during evidence accumulation. eLife 7, e34929 (2018).
Ding, L. & Gold, J. I. Separate, causal roles of the caudate in saccadic choice and execution in a perceptual decision task. Neuron 75, 865–874 (2012).
Znamenskiy, P. & Zador, A. M. Corticostriatal neurons in auditory cortex drive decisions during auditory discrimination. Nature 497, 482–485 (2013).
Guo, L., Walker, W. I., Ponvert, N. D., Penix, P. L. & Jaramillo, S. Stable representation of sounds in the posterior striatum during flexible auditory decisions. Nat. Commun. 9, 1534 (2018).
Forstmann, B. U., Brown, S., Dutilh, G., Neumann, J. & Wagenmakers, E. J. The neural substrate of prior information in perceptual decision making: a model-based analysis. Front. Hum. Neurosci. 4, 40 (2010).
Vilares, I., Howard, J. D., Fernandes, H. L., Gottfried, J. A. & Kording, K. P. Differential representations of prior and likelihood uncertainty in the human brain. Curr. Biol. 22, 1641–1648 (2012).
Horga, G. et al. Dopamine-related disruption of functional topography of striatal connections in unmedicated patients with schizophrenia. JAMA Psychiatry 73, 862–870 (2016).
Bateup, H. S. et al. Distinct subclasses of medium spiny neurons differentially regulate striatal motor behaviors. Proc. Natl Acad. Sci. USA 107, 14845–14850 (2010).
Beeler, J. A. et al. A role for dopamine-mediated learning in the pathophysiology and treatment of Parkinson’s disease. Cell Rep. 2, 1747–1761 (2012).
Durieux, P. F., Schiffmann, S. N. & De Kerchove d’Exaerde, A. Differential regulation of motor control and response to dopaminergic drugs by D1R and D2R neurons in distinct dorsal striatum subregions. EMBO J. 31, 640–653 (2012).
Mowery, T. M. et al. The sensory striatum is permanently impaired by transient developmental deprivation. Cell Rep. 19, 2462–2468 (2017).
Grace, A. A. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat. Rev. Neurosci. 17, 524–532 (2016).
Howes, O. D. & Murray, R. M. Schizophrenia: an integrated sociodevelopmental–cognitive model. Lancet 383, 1677–1687 (2014).
Baker, S. C., Konova, A. B., Daw, N. D. & Horga, G. A distinct inferential mechanism for delusions in schizophrenia. Brain 142, 1797–1812 (2019).
Corlett, P. R. et al. Disrupted prediction-error signal in psychosis: evidence for an associative account of delusions. Brain 130, 2387–2400 (2007).
Davies, D. J., Teufel, C. & Fletcher, P. C. Anomalous perceptions and beliefs are associated with shifts toward different types of prior knowledge in perceptual inference. Schizophr. Bull. 44, 1245–1253 (2018).
Corlett, P. R. et al. Hallucinations and strong priors. Trends Cogn. Sci. 23, 114–127 (2019).
Javitt, D. C. & Sweet, R. A. Auditory dysfunction in schizophrenia: integrating clinical and basic features. Nat. Rev. Neurosci. 16, 535–550 (2015).
Hosoya, T., Baccus, S. A. & Meister, M. Dynamic predictive coding by the retina. Nature 436, 71–77 (2005).
Singla, S., Dempsey, C., Warren, R., Enikolopov, A. G. & Sawtell, N. B. A cerebellum-like circuit in the auditory system cancels responses to self-generated sounds. Nat. Neurosci. 20, 943–950 (2017).
Freedman, R. et al. Neurobiological studies of sensory gating in schizophrenia. Schizophr. Bull. 13, 669–678 (1987).
Jensen, J. & Kapur, S. Salience and psychosis: moving from theory to practise. Psychol. Med. 39, 197–198 (2009).
Feinberg, I. Efference copy and corollary discharge: implications for thinking and its disorders. Schizophr. Bull. 4, 636–640 (1978).
Ford, J. M. & Mathalon, D. H. Corollary discharge dysfunction in schizophrenia: can it explain auditory hallucinations? Int. J. Psychophysiol. 58, 179–189 (2005).
Frith, C. D. The positive and negative symptoms of schizophrenia reflect impairments in the perception and initiation of action. Psychol. Med. 17, 631–648 (1987).
Leptourgos, P., Deneve, S. & Jardri, R. Can circular inference relate the neuropathological and behavioral aspects of schizophrenia? Curr. Opin. Neurobiol. 46, 154–161 (2017).
Gold, J. I. & Shadlen, M. N. Banburismus and the brain: decoding the relationship between sensory stimuli, decisions, and reward. Neuron 36, 299–308 (2002).
Schlack, A. & Albright, T. D. Remembering visual motion: neural correlates of associative plasticity and motion recall in cortical area MT. Neuron 53, 881–890 (2007).
Hanks, T. D., Mazurek, M. E., Kiani, R., Hopp, E. & Shadlen, M. N. Elapsed decision time affects the weighting of prior probability in a perceptual decision task. J. Neurosci. 31, 6339–6352 (2011).
Kok, P., Brouwer, G. J., van Gerven, M. A. & de Lange, F. P. Prior expectations bias sensory representations in visual cortex. J. Neurosci. 33, 16275–16284 (2013).
Summerfield, C. & Egner, T. Expectation (and attention) in visual cognition. Trends Cogn. Sci. 13, 403–409 (2009).
Slifstein, M. et al. Deficits in prefrontal cortical and extrastriatal dopamine release in schizophrenia: a positron emission tomographic functional magnetic resonance imaging study. JAMA Psychiatry 72, 316–324 (2015).
Bao, S., Chan, V. T. & Merzenich, M. M. Cortical remodelling induced by activity of ventral tegmental dopamine neurons. Nature 412, 79–83 (2001).
Chun, S. et al. Specific disruption of thalamic inputs to the auditory cortex in schizophrenia models. Science 344, 1178–1182 (2014).
Gritton, H. J. et al. Cortical cholinergic signaling controls the detection of cues. Proc. Natl Acad. Sci. USA 113, E1089–E1097 (2016).
Kilgard, M. P. & Merzenich, M. M. Cortical map reorganization enabled by nucleus basalis activity. Science 279, 1714–1718 (1998).
Yu, A. J. & Dayan, P. Acetylcholine in cortical inference. Neural Netw. 15, 719–730 (2002).
Iglesias, S. et al. Hierarchical prediction errors in midbrain and basal forebrain during sensory learning. Neuron 80, 519–530 (2013).
Rowntree, D. W., Nevin, S. & Wilson, A. The effects of diisopropylfluorophosphonate in schizophrenia and manic depressive psychosis. J. Neurol. Neurosurg. Psychiatry 13, 47–62 (1950).
Perry, E. K. & Perry, R. H. Acetylcholine and hallucinations: disease-related compared to drug-induced alterations in human consciousness. Brain Cognit. 28, 240–258 (1995).
Buchanan, R. W. et al. Galantamine for the treatment of cognitive impairments in people with schizophrenia. Am. J. Psychiatry 165, 82–89 (2008).
Keefe, R. S. et al. Efficacy and safety of donepezil in patients with schizophrenia or schizoaffective disorder: significant placebo/practice effects in a 12-week, randomized, double-blind, placebo-controlled trial. Neuropsychopharmacology 33, 1217–1228 (2008).
Aston-Jones, G. & Cohen, J. D. An integrative theory of locus coeruleus–norepinephrine function: adaptive gain and optimal performance. Annu. Rev. Neurosci. 28, 403–450 (2005).
Yu, A. J. & Dayan, P. Uncertainty, neuromodulation, and attention. Neuron 46, 681–692 (2005).
Krishnamurthy, K., Nassar, M. R., Sarode, S. & Gold, J. I. Arousal-related adjustments of perceptual biases optimize perception in dynamic environments. Nat. Hum. Behav. 1, 0107 (2017).
Yates, J. L., Park, I. M., Katz, L. N., Pillow, J. W. & Huk, A. C. Functional dissection of signal and noise in MT and LIP during decision-making. Nat. Neurosci. 20, 1285–1292 (2017).
Abi-Dargham, A. et al. Increased striatal dopamine transmission in schizophrenia: confirmation in a second cohort. Am. J. Psychiatry 155, 761–767 (1998).
Acknowledgements
The authors thank T. Maia, S. Haber and K. Schmack for insightful discussions on the ideas presented in this article. The authors acknowledge funding sources: G.H.: R01MH117323, R01MH114965;A.A-D.: R01MH109635.
Peer review information
Nature Reviews Neuroscience thanks P. Corlett, P. Fletcher and the other, anonymous, reviewer(s), for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
G.H. researched data for the article, and both authors made substantial contributions to discussion of the content and writing, reviewing and editing the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Schizophrenia
-
A psychiatric illness characterized by a variety of symptoms, including positive symptoms, negative symptoms (for example, apathy and amotivation) and cognitive impairments (for example, memory deficits).
- Psychotic disorders
-
A group of disorders, including schizophrenia and other disorders, such as bipolar disorder, with psychotic features, that present with psychotic or positive symptoms.
- Auditory verbal hallucinations
-
(AVH). Percepts of speech or voices without corresponding speech stimuli.
- Positive symptoms
-
Also known as psychotic symptoms or psychosis; symptoms that are added to the repertoire of usual experiences and that represent a loss of contact with reality (that is, subjective experiences that substantially deviate from what most perceive as objective evidence), including hallucinations and delusions.
- Prodromal
-
Related to the psychosis prodrome or prodromal phase, terms that refer to the phase preceding the development of full-blown symptoms of a psychotic disorder; typically defined by the expression of attenuated forms of positive symptoms.
- Marr’s three levels of analysis
-
A framework whereby information-processing systems can be understood at three distinct, complementary levels: computational (the problem that is solved), algorithmic (what representations and processes are used to solve this problem) and implementational (the physical and biological substrates through which the solution is realized).
- Bayesian inference
-
A statistical algorithm for probabilistic estimation that relies on the optimal combination of prior knowledge and new data.
- Inner speech
-
A person’s inner dialogue, expressed as a silent conscious stream of thoughts in a coherent linguistic form.
- Gating
-
A process by which the passage of information is actively controlled, thereby facilitating or impeding information flow.
Rights and permissions
About this article
Cite this article
Horga, G., Abi-Dargham, A. An integrative framework for perceptual disturbances in psychosis. Nat Rev Neurosci 20, 763–778 (2019). https://doi.org/10.1038/s41583-019-0234-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41583-019-0234-1
This article is cited by
-
A unifying theory explains seemingly contradictory biases in perceptual estimation
Nature Neuroscience (2024)
-
Prior experience modifies acquisition trajectories via response–strategy sampling
Animal Cognition (2023)
-
Function and therapeutic value of astrocytes in neurological diseases
Nature Reviews Drug Discovery (2022)
-
A model for learning based on the joint estimation of stochasticity and volatility
Nature Communications (2021)
-
Precision weighting of cortical unsigned prediction error signals benefits learning, is mediated by dopamine, and is impaired in psychosis
Molecular Psychiatry (2021)