Abstract
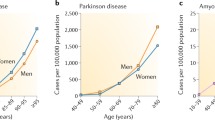
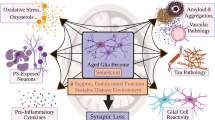
Globally, 50 million people live with dementia, with Alzheimer disease (AD) being responsible for two-thirds of the total cases. As ageing is the main risk factor for dementia-related neurodegeneration, changes in the timing or nature of the cellular hallmarks of normal ageing might be key to understanding the events that convert normal ageing into neurodegeneration. Cellular senescence is a candidate mechanism that might be important for this conversion. Under persistent stress, as occurs in ageing, both postmitotic cells — including neurons — and proliferative cells — such as astrocytes and microglia, among others — can engender a state of chronic cellular senescence that is characterized by the secretion of pro-inflammatory molecules that promote the functional decline of tissues and organs. Ablation of senescent cells has been postulated as a promising therapeutic venue to target the ageing phenotype and, thus, prevent or mitigate ageing-related diseases. However, owing to a lack of evidence, it is not possible to label cellular senescence as a cause or a consequence of neurodegeneration. This Review examines cellular senescence in the context of ageing and AD, and discusses which of the processes — cellular senescence or AD — might come first.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
13 August 2020
A Correction to this paper has been published: https://doi.org/10.1038/s41583-020-0366-3
References
Wortmann, M. World Alzheimer Report 2014: dementia and risk reduction. Alzheimer’s Dement. 11, P837 (2015).
Winblad, B. et al. Defeating Alzheimer’s disease and other dementias: a priority for European science and society. Lancet Neurol. 15, 455–532 (2016).
Ritchie, K. & Lovestone, S. The dementias. Lancet 360, 1759–1766 (2002).
Corriveau, R. A. et al. Alzheimer’s Disease-Related Dementias Summit 2016: national research priorities. Neurology 89, 2381–2391 (2017).
Hou, Y. et al. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 15, 565–581 (2019).
Soto, C. & Pritzkow, S. Protein misfolding, aggregation, and conformational strains in neurodegenerative diseases. Nat. Neurosci. 21, 1332–1340 (2018).
Golde, T. E., Borchelt, D. R., Giasson, B. I. & Lewis, J. Thinking laterally about neurodegenerative proteinopathies. J. Clin. Invest. 123, 1847–1855 (2013).
Yen, S. H., Dickson, D. W., Crowe, A., Butler, M. & Shelanski, M. L. Alzheimer’s neurofibrillary tangles contain unique epitopes and epitopes in common with the heat-stable microtubule associated proteins tau and MAP2. Am. J. Pathol. 126, 81–91 (1987).
Grundke-Iqbal, I. et al. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc. Natl Acad. Sci. USA 83, 4913–4917 (1986).
Kosik, K. S., Joachim, C. L. & Selkoe, D. J. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc. Natl Acad. Sci. USA 83, 4044–4048 (1986).
Glenner, G. G. & Wong, C. W. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 120, 885–890 (1984).
Masters, C. L. et al. Amyloid plaque core protein in Alzheimer disease and down syndrome. Proc. Natl Acad. Sci. USA 82, 4245–4249 (1985).
Spillantini, M. G. et al. α-Synuclein in Lewy bodies. Nature 388, 839–840 (1997).
Arai, T. et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem. Biophys. Res. Commun. 351, 602–611 (2006).
Neumann, M. et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314, 130–133 (2006).
Elobeid, A., Libard, S., Leino, M., Popova, S. N. & Alafuzoff, I. Altered proteins in the aging brain. J. Neuropathol. Exp. Neurol. 75, 316–325 (2016).
Wyss-Coray, T. Ageing, neurodegeneration and brain rejuvenation. Nature 539, 180–186 (2016).
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013).
Kennedy, B. K. et al. Geroscience: linking aging to chronic disease. Cell 159, 709–713 (2014).
Baker, D. J. & Petersen, R. C. Cellular senescence in brain aging and neurodegenerative diseases: evidence and perspectives. J. Clin. Invest. 128, 1208–1216 (2018).
Hayflick, L. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 37, 614–636 (1965).
Kuilman, T., Michaloglou, C., Mooi, W. J. & Peeper, D. S. The essence of senescence. Genes. Dev. 24, 2463–2479 (2010).
Coppé, J.-P. et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 6, 2853–2868 (2008).
van Deursen, J. M. The role of senescent cells in ageing. Nature 509, 439–446 (2014).
Salminen, A., Kauppinen, A. & Kaarniranta, K. Emerging role of NF-κB signaling in the induction of senescence-associated secretory phenotype (SASP). Cell. Signal. 24, 835–845 (2012).
Bussian, T. J. et al. Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature 562, 578–582 (2018). This research paper shows that clearance of senescent astrocytes and microglia in a mouse model of tauopathy (MAPTP301SPS19 mice) alleviates NFT formation and gliosis with a consequent improvement in cognition.
Musi, N. et al. Tau protein aggregation is associated with cellular senescence in the brain. Aging Cell 17, e12840 (2018). This article finds that the formation of tau-containing NFTs activates senescence in both human and mouse NFT-containing neurons.
Zhang, P. et al. Senolytic therapy alleviates Aβ-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer’s disease model. Nat. Neurosci. 22, 719–728 (2019). This paper shows that senescent OPCs accumulate around amyloid plaque deposition in samples of mice and human brains with AD.
Tan, F. C. C., Hutchison, E. R., Eitan, E. & Mattson, M. P. Are there roles for brain cell senescence in aging and neurodegenerative disorders? Biogerontology 15, 643–660 (2014).
Galluzzi, L. et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 25, 486–541 (2018).
Tavana, O. et al. Absence of p53-dependent apoptosis leads to UV radiation hypersensitivity, enhanced immunosuppression and cellular senescence. Cell Cycle 9, 3328–3336 (2010).
Chen, Q. M., Juping, L. I. U. & Merrett, J. B. Apoptosis or senescence-like growth arrest: influence of cell-cycle position, p53, p21 and bax in H2O2 response of normal human fibroblasts. Biochem. J. 347, 543–551 (2000).
Hayflick, L. & Moorhead, P. S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 25, 585–621 (1961).
Song, Y. S., Lee, B. Y. & Hwang, E. S. Dinstinct ROS and biochemical profiles in cells undergoing DNA damage-induced senescence and apoptosis. Mech. Ageing Dev. 126, 580–590 (2005).
Spallarossa, P. et al. Doxorubicin induces senescence or apoptosis in rat neonatal cardiomyocytes by regulating the expression levels of the telomere binding factors 1 and 2. Am. J. Physiol. Heart Circ. Physiol. 297, H2169–H2181 (2009).
Kuilman, T. et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 133, 1019–1031 (2008).
Özcan, S. et al. Unbiased analysis of senescence associated secretory phenotype (SASP) to identify common components following different genotoxic stresses. Aging 8, 1316–1329 (2016).
Coppé, J.-P., Desprez, P.-Y., Krtolica, A. & Campisi, J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol. 5, 99–118 (2010).
Xue, W. et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 445, 656–660 (2007).
Freund, A., Orjalo, A. V., Desprez, P.-Y. & Campisi, J. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol. Med. 16, 238–246 (2010).
Ovadya, Y. et al. Impaired immune surveillance accelerates accumulation of senescent cells and aging. Nat. Commun. 9, 5435 (2018). This article provides evidence to support the notion that impaired immune surveillance contributes to the accumulation of senescent cells in ageing.
Lanna, A., Henson, S. M., Escors, D. & Akbar, A. N. The kinase p38 activated by the metabolic regulator AMPK and scaffold TAB1 drives the senescence of human T cells. Nat. Immunol. 15, 965–972 (2014).
Wang, A. S., Ong, P. F., Chojnowski, A., Clavel, C. & Dreesen, O. Loss of lamin B1 is a biomarker to quantify cellular senescence in photoaged skin. Sci. Rep. 7, 15678 (2017).
Helman, A. et al. p16Ink4a-induced senescence of pancreatic β cells enhances insulin secretion. Nat. Med. 22, 412–420 (2016).
Thompson, P. J. et al. Targeted elimination of senescent β cells prevents type 1 diabetes. Cell Metab. 29, e10 (2019).
He, S. & Sharpless, N. E. Senescence in health and disease. Cell 169, 1000–1011 (2017).
Riessland, M. et al. Loss of SATB1 induces p21-dependent cellular senescence in post-mitotic dopaminergic neurons. Cell Stem Cell 25, 514–530.e8 (2019). This paper finds that the expression of SATB1, a genetic risk factor in Parkinson disease that encodes a regulator of senescence in dopaminergic neurons specifically, is reduced in the substantia nigra of brain tissue from individuals with sporadic Parkinson disease.
Arendt, T., Holzer, M. & Gärtner, U. Neuronal expression of cycline dependent kinase inhibitors of the INK4 family in Alzheimer’s disease. J. Neural Transm. 105, 949–960 (1998).
Gärtner, U., Holzer, M., Heumann, R. & Arendt, T. Induction of p21ras in Alzheimer pathology. NeuroReport 6, 1441–1444 (1995).
Anderson, R. et al. Length-independent telomere damage drives post-mitotic cardiomyocyte senescence. EMBO J. 38, e100492 (2019).
Maejima, Y., Adachi, S., Ito, H., Hirao, K. & Isobe, M. Induction of premature senescence in cardiomyocytes by doxorubicin as a novel mechanism of myocardial damage. Aging Cell 7, 125–136 (2008).
Gorissen, B. et al. Hypoxia negatively affects senescence in osteoclasts and delays osteoclastogenesis. J. Cell. Physiol. 234, 414–426 (2018).
Farr, J. N. et al. Identification of senescent cells in the bone microenvironment. J. Bone Miner. Res. 31, 1920–1929 (2016).
Storer, M. et al. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell 155, 1119–1130 (2013).
Demaria, M. et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell 31, 722–733 (2014).
Campisi, J. Cellular senescence as a tumor-suppressor mechanism. Trends Cell Biol. 11, S27–S31 (2001).
Serrano, M., Lin, A. W., McCurrach, M. E., Beach, D. & Lowe, S. W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88, 593–602 (1997).
Rodríguez, J. A. et al. Antagonistic pleiotropy and mutation accumulation influence human senescence and disease. Nat. Ecol. Evol. 1, 55 (2017).
Sharpless, N. E. & Sherr, C. J. Forging a signature of in vivo senescence. Nat. Rev. Cancer 15, 397–408 (2015).
Hernandez-Segura, A. et al. Unmasking transcriptional heterogeneity in senescent cells. Curr. Biol. 27, 2652–2660.e4 (2017).
Herbig, U., Jobling, W. A., Chen, B. P. C., Chen, D. J. & Sedivy, J. M. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21CIP1, but not p16INK4a. Mol. Cell 14, 501–513 (2004).
Bodnar, A. G. et al. Extension of life-span by introduction of telomerase into normal human cells. Science 279, 349–352 (1998).
Sedelnikova, O. A. et al. Senescing human cells and ageing mice accumulate DNA lesions with unrepairable double-strand breaks. Nat. Cell Biol. 6, 168–170 (2004).
Stöckl, P., Hütter, E., Zwerschke, W. & Jansen-Dürr, P. Sustained inhibition of oxidative phosphorylation impairs cell proliferation and induces premature senescence in human fibroblasts. Exp. Gerontol. 41, 674–682 (2006).
Sasaki, M., Kajiya, H., Ozeki, S., Okabe, K. & Ikebe, T. Reactive oxygen species promotes cellular senescence in normal human epidermal keratinocytes through epigenetic regulation of p16INK4a. Biochem. Biophys. Res. Commun. 452, 622–628 (2014).
Gorgoulis, V. G. & Halazonetis, T. D. Oncogene-induced senescence: the bright and dark side of the response. Curr. Opin. Cell Biol. 22, 816–827 (2010).
Sabin, R. J. & Anderson, R. M. Cellular senescence — its role in cancer and the response to ionizing radiation. Genome Integr. 2, 7 (2011).
Chapman, J., Fielder, E. & Passos, J. F. Mitochondrial dysfunction and cell senescence: deciphering a complex relationship. FEBS Lett. 593, 1566–1579 (2019).
Safaiyan, S. et al. Age-related myelin degradation burdens the clearance function of microglia during aging. Nat. Neurosci. 19, 995–998 (2016). This paper shows that age-dependent myelin fragmentation could contribute to the overload of microglial lysosomes, and that these microglial cells exhibit markers of senescence.
Caldeira, C. et al. Key aging-associated alterations in primary microglia response to β-amyloid stimulation. Front. Aging Neurosci. 9, 277 (2017).
Wiley, C. D. et al. Mitochondrial dysfunction induces senescence with a distinct secretory phenotype. Cell Metab. 23, 303–314 (2016). This paper shows that mitochondrial impairment is capable of inducing mitochondrial dysfunction-associated senescence, which causes growth arrest and is associated with an SASP of a particular composition.
d’Adda di Fagagna, F. Living on a break: cellular senescence as a DNA-damage response. Nat. Rev. Cancer 8, 512–522 (2008).
Burma, S., Chen, B. P., Murphy, M., Kurimasa, A. & Chen, D. J. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J. Biol. Chem. 276, 42462–42467 (2001).
Lou, Z., Minter-Dykhouse, K., Wu, X. & Chen, J. MDC1 is coupled to activated CHK2 in mammalian DNA damage response pathways. Nature 421, 957–961 (2003).
Stucki, M. et al. MDC1 directly binds phosphorylated histone H2AX to regulate cellular responses to DNA double-strand breaks. Cell 123, 1213–1226 (2005).
Huyen, Y. et al. Methylated lysine 79 of histone H3 targets 53BP1 to DNA double-strand breaks. Nature 432, 406–411 (2004).
Buscemi, G. et al. Activation of ATM and Chk2 kinases in relation to the amount of DNA strand breaks. Oncogene 23, 7691–7700 (2004).
Smits, V. A. J., Reaper, P. M. & Jackson, S. P. Rapid PIKK-dependent release of Chk1 from chromatin promotes the DNA-damage checkpoint response. Curr. Biol. 16, 150–159 (2006).
Martínez-Zamudio, R. I., Robinson, L., Roux, P.-F. & Bischof, O. SnapShot: cellular senescence pathways. Cell 170, 816–816.e1 (2017).
Herranz, N. & Gil, J. Mechanisms and functions of cellular senescence. J. Clin. Invest. 128, 1238–1246 (2018).
Bracken, A. P. et al. The Polycomb group proteins bind throughout the INK4A–ARF locus and are disassociated in senescent cells. Genes. Dev. 21, 525–530 (2007).
Gil, J. & Peters, G. Regulation of the INK4b–ARF–INK4a tumour suppressor locus: all for one or one for all. Nat. Rev. Mol. Cell Biol. 7, 667–677 (2006).
Lee, S. & Schmitt, C. A. The dynamic nature of senescence in cancer. Nat. Cell Biol. 21, 94–101 (2019).
Krishnamurthy, J. et al. Ink4a/Arf expression is a biomarker of aging. J. Clin. Invest. 114, 1299–1307 (2004).
Liu, J.-Y. et al. Cells exhibiting strong p16INK4a promoter activation in vivo display features of senescence. Proc. Natl Acad. Sci. USA 116, 2603–2611 (2019).
Chkhotua, A. B. et al. Increased expression of p16INK4a and p27Kip1 cyclin-dependent kinase inhibitor genes in aging human kidney and chronic allograft nephropathy. Am. J. Kidney Dis. 41, 1303–1313 (2003).
Kajstura, J. et al. Telomere shortening is an in vivo marker of myocyte replication and aging. Am. J. Pathol. 156, 813–819 (2000).
Hsu, C.-H., Altschuler, S. J. & Wu, L. F. Patterns of early p21 dynamics determine proliferation–senescence cell fate after chemotherapy. Cell 178, 361–373.e12 (2019).
Childs, B. G., Durik, M., Baker, D. J. & van Deursen, J. M. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat. Med. 21, 1424–1435 (2015).
Dimri, G. P. et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 92, 9363–9367 (1995).
Acosta, J. C. et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 15, 978–990 (2013).
da Silva, P. F. L. et al. The bystander effect contributes to the accumulation of senescent cells in vivo. Aging Cell 18, e12848 (2019).
Biran, A. et al. Senescent cells communicate via intercellular protein transfer. Genes Dev. 29, 791–802 (2015).
Nelson, G. et al. A senescent cell bystander effect: senescence-induced senescence. Aging Cell 11, 345–349 (2012).
Faget, D. V., Ren, Q. & Stewart, S. A. Unmasking senescence: context-dependent effects of SASP in cancer. Nat. Rev. Cancer 19, 439–453 (2019).
Sanada, F. et al. Source of chronic inflammation in aging. Front. Cardiovasc. Med. 5, 12 (2018).
Franceschi, C. et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 908, 244–254 (2000).
McHugh, D. & Gil, J. Senescence and aging: causes, consequences, and therapeutic avenues. J. Cell Biol. 217, 65–77 (2018).
Weirich-Schwaiger, H., Weirich, H. G., Gruber, B., Schweiger, M. & Hirsch-Kauffman, M. Correlation between senescence and DNA repair in cells from young and old individuals and in premature aging syndromes. Mutat. Res. 316, 37–48 (1994).
Baker, D. J. et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479, 232–236 (2011). This paper shows that the conditional and selective elimination of senescent cells expressing p16INK4a delays the premature ageing phenotype in BubR1-insuficient mice.
Hanks, S. et al. Constitutional aneuploidy and cancer predisposition caused by biallelic mutations in BUB1B. Nat. Genet. 36, 1159–1161 (2004).
Kulukian, A., Han, J. S. & Cleveland, D. W. Unattached kinetochores catalyze production of an anaphase inhibitor that requires a Mad2 template to prime Cdc20 for BubR1 binding. Dev. Cell 16, 105–117 (2009).
Baker, D. J. et al. BubR1 insufficiency causes early onset of aging-associated phenotypes and infertility in mice. Nat. Genet. 36, 744–749 (2004).
Chang, J. et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med. 22, 78–83 (2016). This paper describes a senolytic compound named ABT263 (an inhibitor of the anti-apoptotic proteins BCL-2 and BCL-xL). The authors use ABT263 in a genotoxic stress-induced mouse model to pharmacologically eliminate senescent cells, which results in a delay in the emergence of the ageing phenotype.
Trifunovic, A. et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429, 417–423 (2004).
Baker, D. J. et al. Naturally occurring p16Ink4a-positive cells shorten healthy lifespan. Nature 530, 184–189 (2016). This paper studies the physiological contribution of the natural accumulation of senescent cells to ageing-related phenotypes in aged mice. Interestingly, elimination of senescent cells improves aged-related cognitive decline in older mice.
Niccoli, T. & Partridge, L. Ageing as a risk factor for disease. Curr. Biol. 22, R741–R752 (2012).
McShea, A., Harris, P. L., Webster, K. R., Wahl, A. F. & Smith, M. A. Abnormal expression of the cell cycle regulators P16 and CDK4 in Alzheimer’s disease. Am. J. Pathol. 150, 1933–1939 (1997).
Bhat, R. et al. Astrocyte senescence as a component of Alzheimer’s disease. PLoS ONE 7, e45069 (2012).
Calignon, A. de et al. Tangle-bearing neurons survive despite disruption of membrane integrity in a mouse model of tauopathy. J. Neuropathol. Exp. Neurol. 68, 757–761 (2009).
Ramsden, M. Age-dependent neurofibrillary tangle formation, neuron loss, and memory impairment in a mouse model of human tauopathy (P301L). J. Neurosci. 25, 10637–10647 (2005).
SantaCruz, K. Tau suppression in a neurodegenerative mouse model improves memory function. Science 309, 476–481 (2005).
Arendt, T., Rödel, L., Gärtner, U. & Holzer, M. Expression of the cyclin-dependent kinase inhibitor p16 in Alzheimer’s disease. Neuroreport 7, 3047–3049 (1996).
McShea, A., Wahl, A. F. & Smith, M. A. Re-entry into the cell cycle: a mechanism for neurodegeneration in Alzheimer disease. Med. Hypotheses 52, 525–527 (1999).
Alonso, A. D., Grundke-Iqbal, I., Barra, H. S. & Iqbal, K. Abnormal phosphorylation of tau and the mechanism of Alzheimer neurofibrillary degeneration: sequestration of microtubule-associated proteins 1 and 2 and the disassembly of microtubules by the abnormal tau. Proc. Natl Acad. Sci. USA 94, 298–303 (1997).
Borchelt, D. R. et al. Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant presenilin 1 and amyloid precursor proteins. Neuron 19, 939–945 (1997).
Laurent, C., Buée, L. & Blum, D. Tau and neuroinflammation: what impact for Alzheimer’s disease and tauopathies? Biomed. J. 41, 21–33 (2018).
Yoshiyama, Y. et al. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 53, 337–351 (2007).
ADAPT Research Group et al. Cognitive function over time in the Alzheimer’s Disease Anti-inflammatory Prevention Trial (ADAPT): results of a randomized, controlled trial of naproxen and celecoxib. Arch. Neurol. 65, 896–905 (2008).
Meyer, P.-F. et al. INTREPAD: a randomized trial of naproxen to slow progress of presymptomatic Alzheimer disease. Neurology 92, e2070–e2080 (2019).
Streit, W. J. Microglial senescence: does the brain’s immune system have an expiration date? Trends Neurosci. 29, 506–510 (2006).
Streit, W. J., Braak, H., Xue, Q.-S. & Bechmann, I. Dystrophic (senescent) rather than activated microglial cells are associated with tau pathology and likely precede neurodegeneration in Alzheimer’s disease. Acta Neuropathol. 118, 475–485 (2009).
Glass, C. K., Saijo, K., Winner, B., Marchetto, M. C. & Gage, F. H. Mechanisms underlying inflammation in neurodegeneration. Cell 140, 918–934 (2010).
Loy, C. T., Schofield, P. R., Turner, A. M. & Kwok, J. B. J. Genetics of dementia. Lancet 383, 828–840 (2014).
Kunkle, B. W. et al. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat. Genet. 51, 414–430 (2019).
Jansen, I. E. et al. Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer’s disease risk. Nat. Genet. 51, 404–413 (2019).
Scheltens, P. et al. Alzheimer’s disease. Lancet 388, 505–517 (2016).
Folk, W. P. et al. Loss of the tumor suppressor BIN1 enables ATM Ser/Thr kinase activation by the nuclear protein E2F1 and renders cancer cells resistant to cisplatin. J. Biol. Chem. 294, 5700–5719 (2019).
Zingoni, A. et al. Genotoxic stress induces senescence-associated ADAM10-dependent release of NKG2D MIC ligands in multiple myeloma cells. J. Immunol. 195, 736–748 (2015).
Vinatier, C., Domínguez, E., Guicheux, J. & Caramés, B. Role of the inflammation–autophagy–senescence integrative network in osteoarthritis. Front. Physiol. 9, 706 (2018).
Sierksma, A. et al. Novel Alzheimer risk genes determine the microglia response to amyloid-β but not to TAU pathology. EMBO Mol. Med. 12, e10606 (2020).
Soreq, L. et al. Major shifts in glial regional identity are a transcriptional hallmark of human brain aging. Cell Rep. 18, 557–570 (2017).
Mi, S. et al. LINGO-1 negatively regulates myelination by oligodendrocytes. Nat. Neurosci. 8, 745–751 (2005).
Bartzokis, G. Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer’s disease. Neurobiol. Aging 25, 5–18 (2004).
Ou-Yang, M.-H. & Van Nostrand, W. E. The absence of myelin basic protein promotes neuroinflammation and reduces amyloid β-protein accumulation in Tg-5xFAD mice. J. Neuroinflammation 10, 134 (2013).
Liao, M.-C., Ahmed, M., Smith, S. O. & Van Nostrand, W. E. Degradation of amyloid β protein by purified myelin basic protein. J. Biol. Chem. 284, 28917–28925 (2009).
Graeber, M. B. et al. Rediscovery of the case described by Alois Alzheimer in 1911: historical, histological and molecular genetic analysis. Neurogenetics 1, 73–80 (1997).
Dean, D. C. III et al. Association of amyloid pathology with myelin alteration in preclinical Alzheimer disease. JAMA Neurol. 74, 41–49 (2017). This study shows that asymptomatic individuals carrying genetic risk factors for AD present myelin abnormalities in the white matter, indicating that myelin disruption may represent an early feature of the disease process.
Strittmatter, W. J. & Roses, A. D. Apolipoprotein E and Alzheimer disease. Proc. Natl Acad. Sci. USA 92, 4725–4727 (1995).
Bartzokis, G. et al. Apolipoprotein E affects both myelin breakdown and cognition: implications for age-related trajectories of decline into dementia. Biol. Psychiatry 62, 1380–1387 (2007).
Dean, D. C. III et al. Brain differences in infants at differential genetic risk for late-onset Alzheimer disease: a cross-sectional imaging study. JAMA Neurol. 71, 11–22 (2014). This paper shows that a reduction of the myelin water fraction is observable at an early developmental stage in infants carrying the APOE ε4 allele.
Franklin, R. J. M. & ffrench-Constant, C. Regenerating CNS myelin — from mechanisms to experimental medicines. Nat. Rev. Neurosci. 18, 753–769 (2017).
Lloyd, A. F. & Miron, V. E. The pro-remyelination properties of microglia in the central nervous system. Nat. Rev. Neurol. 15, 447–458 (2019).
Neumann, B. et al. Metformin restores CNS remyelination capacity by rejuvenating aged stem cells. Cell Stem Cell 25, 473–485.e8 (2019).
Flanary, B. E., Sammons, N. W., Nguyen, C., Walker, D. & Streit, W. J. Evidence that aging and amyloid promote microglial cell senescence. Rejuvenation Res. 10, 61–74 (2007).
Gold, B. T., Johnson, N. F., Powell, D. K. & Smith, C. D. White matter integrity and vulnerability to Alzheimer’s disease: preliminary findings and future directions. Biochim. Biophys. Acta 1822, 416–422 (2012).
Carmona, S. et al. The role of TREM2 in Alzheimer’s disease and other neurodegenerative disorders. Lancet Neurol. 17, 721–730 (2018).
Poliani, P. L. et al. TREM2 sustains microglial expansion during aging and response to demyelination. J. Clin. Invest. 125, 2161–2170 (2015).
Hardy, J. & Higgins, G. Alzheimer’s disease: the amyloid cascade hypothesis. Science 256, 184–185 (1992).
Hardy, J. Alzheimer’s disease: the amyloid cascade hypothesis: an update and reappraisal. J. Alzheimer’s Dis. 9, 151–153 (2006).
Krasemann, S. et al. The TREM2–APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity 47, 566–581.e9 (2017).
Keren-Shaul, H. et al. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169, 1276–1290.e17 (2017).
Streit, W. J., Sammons, N. W., Kuhns, A. J. & Larry Sparks, D. Dystrophic microglia in the aging human brain. Glia 45, 208–212 (2004).
Hunter, S., Arendt, T. & Brayne, C. The senescence hypothesis of disease progression in Alzheimer disease: an integrated matrix of disease pathways for FAD and SAD. Mol. Neurobiol. 48, 556–570 (2013).
Xu, M. et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 24, 1246–1256 (2018).
Zhu, Y. et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 14, 644–658 (2015).
Wang, E. Senescent human fibroblasts resist programmed cell death, and failure to suppress bcl2 is involved. Cancer Res. 55, 2284–2292 (1995).
Sasaki, M., Kumazaki, T., Takano, H., Nishiyama, M. & Mitsui, Y. Senescent cells are resistant to death despite low Bcl-2 level. Mech. Ageing Dev. 122, 1695–1706 (2001).
Ovadya, Y. & Krizhanovsky, V. Strategies targeting cellular senescence. J. Clin. Invest. 128, 1247–1254 (2018).
Yosef, R. et al. Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat. Commun. 7, 11190 (2016). This research paper observes that upregulation of BCL-W and BCL-xL is responsible for the apoptosis resistance of senescent cells.
Sikora, E., Bielak-Zmijewska, A. & Mosieniak, G. Targeting normal and cancer senescent cells as a strategy of senotherapy. Ageing Res. Rev. 55, 100941 (2019).
Currais, A. et al. Fisetin reduces the impact of aging on behavior and physiology in the rapidly aging SAMP8 mouse. J. Gerontol. A Biol. Sci. Med. Sci. 73, 299–307 (2018).
Farr, J. N. et al. Targeting cellular senescence prevents age-related bone loss in mice. Nat. Med. 23, 1072–1079 (2017).
Ogrodnik, M. et al. Cellular senescence drives age-dependent hepatic steatosis. Nat. Commun. 8, 15691 (2017).
Hickson, L. J. et al. Senolytics decrease senescent cells in humans: preliminary report from a clinical trial of dasatinib plus quercetin in individuals with diabetic kidney disease. EBioMedicine 47, 446–456 (2019). This paper presents the first clinical trial of the senolytic compounds dasatinib plus quercetin. The combination of the two reduces the levels of adipose tissue senescent cells and circulating SASP factors in individuals with diabetic kidney disease.
Kirkland, J. L. & Tchkonia, T. Cellular senescence: a translational perspective. EBioMedicine 21, 21–28 (2017).
Acknowledgements
This work was supported in part by the Intramural Research Program of the NIH, National Institute on Ageing.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- INK4–ARF locus
-
A locus containing two genes, CDKN2A and CDKN2B. CDKN2A encodes two proteins, p16INK4a and ARF (known as p14ARF in humans and p19ARF in mice). Both proteins are cell cycle regulators that act as tumour suppressors and play an essential role in the induction and maintenance of senescence. CDKN2B encodes p15INK4b, a cyclin-dependent kinase inhibitor.
- Sterile inflammation
-
A pathogen-free inflammatory process that can be triggered by an acute stimulus, such as ischaemia reperfusion injury, trauma or toxin exposure, or a chronic stimulus, as occurs in chronic diseases and ageing. Damaged cells produce and release damage-associated molecular patterns that activate innate immune cells, which release cytokines and chemokines, further activating an adaptive immune response. Unresolved and prolonged sterile inflammation is detrimental and contributes to ageing.
- Inflammaging
-
Chronic and low-grade inflammation that is associated with ageing and contributes to the pathology of age-related disease. Three main stimuli sustain inflammaging: cell debris accumulation, microbial products from human microbiota and cellular senescence.
- Senolytic
-
A small molecule that selectively eliminates senescent cells. The majority of senolytic compounds aim to target the anti-apoptotic members of the B cell lymphoma 2 (BCL-2) protein family, as they have been shown to be upregulated in senescent cells. Other senolytic strategies have explored impeding p53 activation, by blocking either the interaction of the E3 ubiquitin ligase MDM2 with p53 or the interaction of p53 with forkhead box protein O4 (FOXO4). More recently, drugs have been designed that get converted into cytotoxic compounds in senescent cells, inducing apoptosis, through cleavage by lysosomal β-galactosidase.
- Mitochondrial dysfunction-associated senescence
-
A particular type of senescence observed in vitro and in vivo that is triggered by mitochondrial DNA damage. It involves activation of AMPK and subsequent activation of p53. Senescent cells induced by mitochondrial dysfunction-associated senescence exhibit a senescence-associated secretory phenotype, although it lacks IL-1-dependent factors.
Rights and permissions
About this article
Cite this article
Saez-Atienzar, S., Masliah, E. Cellular senescence and Alzheimer disease: the egg and the chicken scenario. Nat Rev Neurosci 21, 433–444 (2020). https://doi.org/10.1038/s41583-020-0325-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41583-020-0325-z
This article is cited by
-
The interaction between ageing and Alzheimer's disease: insights from the hallmarks of ageing
Translational Neurodegeneration (2024)
-
The Role of Glia Telomere Dysfunction in the Pathogenesis of Central Nervous System Diseases
Molecular Neurobiology (2024)
-
H3K18 lactylation of senescent microglia potentiates brain aging and Alzheimer's disease through the NFκB signaling pathway
Journal of Neuroinflammation (2023)
-
Oligodendrocyte progenitor cells in Alzheimer’s disease: from physiology to pathology
Translational Neurodegeneration (2023)
-
Links between COVID-19 and Parkinson’s disease/Alzheimer’s disease: reciprocal impacts, medical care strategies and underlying mechanisms
Translational Neurodegeneration (2023)