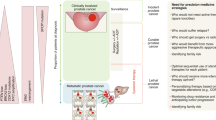
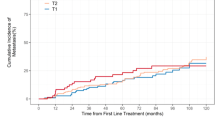
Abstract
Liver metastases from prostate cancer are associated with an aggressive disease course and poor prognosis. Results from autopsy studies indicate a liver metastasis prevalence of up to 25% in patients with advanced prostate cancer. Population data estimate that ~3–10% of patients with metastatic castration-resistant prostate cancer harbour liver metastases at the baseline, rising to 20–30% in post-treatment cohorts, suggesting that selective pressure imposed by novel therapies might promote metastatic spread to the liver. Liver metastases are associated with more aggressive tumour biology than lung metastases. Molecular profiling of liver lesions showed an enrichment of low androgen receptor, neuroendocrine phenotypes and high genomic instability. Despite advancements in molecular imaging modalities such as prostate-specific membrane antigen PET–CT, and liquid biopsy markers such as circulating tumour DNA, early detection of liver metastases from prostate cancer remains challenging, as both approaches are hampered by false positive and false negative results, impeding the accurate identification of early liver lesions. Current therapeutic strategies showed limited efficacy in this patient population. Emerging targeted radionuclide therapies, metastasis-directed therapy, and novel systemic agents have shown preliminary activity against liver metastases, but require further validation. Treatment with various novel prostate cancer therapies might lead to an increase in the prevalence of liver metastasis, underscoring the urgent need for coordinated efforts across preclinical and clinical researchers to improve characterization, monitoring, and management of liver metastases from prostate cancer. Elucidating molecular drivers of liver tropism and interactions with the liver microenvironment might ultimately help to identify actionable targets to enhance survival in this high-risk patient group.
Key points
-
The prevalence of liver metastasis increases in correlation with the progression of prostate cancer, escalating from ~3% in initial metastatic hormone-sensitive prostate cancer (mHSPC) to over 20% in post-therapeutic metastatic castration-resistant prostate cancer (mCRPC). Furthermore, the ratio of liver to lung metastasis also shifts from 1:3 in initial mHSPC to over 1:1 in post-therapeutic mCRPC.
-
The manifestation of liver metastases confers a dismal prognosis, approximately doubling the risk of death compared with other metastatic locations.
-
Liver metastasis progression involves changes in cancer cell phenotype (such as low androgen receptor, neuroendocrine conversion and increased genomic instability), organotropism factors, pre-metastatic niche elements (including liver injury), and intricate interactions with the hepatic microenvironment.
-
Novel molecular imaging such as prostate-specific membrane antigen (PSMA) PET–CT and liquid biopsy have greatly improved liver metastasis detection, but occult liver metastases remain common, as PSMA-PET–CT is still associated with a false-negative rate of ~20% for liver lesions. However, most PSMA-negative liver metastases were shown to be fluorodeoxyglucose-positive and could be successfully detected using fluorodeoxyglucose PET–CT.
-
Current therapies, including androgen deprivation therapy, novel hormone therapies, chemotherapy, and radioligand therapy showed limited efficacy in patients with liver metastases. However, novel molecular targeted radionuclide therapy, metastasis-directed therapy and immunological combination therapy have shown promising preliminary activity against liver metastases.
-
The establishment of multi-omics databases, animal models, real-time monitoring techniques, and the exploration of therapeutic strategies tailored to target specific molecular subtypes of liver metastases should be prioritized in future research.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Siegel, R. L., Miller, K. D., Wagle, N. S. & Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 73, 17–48 (2023).
Ma, B., Wells, A., Wei, L. & Zheng, J. Prostate cancer liver metastasis: dormancy and resistance to therapy. Semin. Cancer Biol. 71, 2–9 (2021).
Tsilimigras, D. I. et al. Liver metastases. Nat. Rev. Dis. Prim. 7, 27 (2021).
Gandaglia, G. et al. Impact of the site of metastases on survival in patients with metastatic prostate cancer. Eur. Urol. 68, 325–334 (2015).
Pond, G. R. et al. The prognostic importance of metastatic site in men with metastatic castration-resistant prostate cancer. Eur. Urol. 65, 3–6 (2014).
Halabi, S. et al. Meta-analysis evaluating the impact of site of metastasis on overall survival in men with castration-resistant prostate cancer. J. Clin. Oncol. 34, 1652–1659 (2016).
Heck, M. M. et al. Treatment outcome, toxicity, and predictive factors for radioligand therapy with (177)Lu-PSMA-I&T in metastatic castration-resistant prostate cancer. Eur. Urol. 75, 920–926 (2019).
Rahbar, K. et al. PSMA targeted radioligand therapy in metastatic castration resistant prostate cancer after chemotherapy, abiraterone and/or enzalutamide. A retrospective analysis of overall survival. Eur. J. Nucl. Med. Mol. Imaging 45, 12–19 (2018).
Beltran, H. et al. A phase II trial of the Aurora kinase A inhibitor alisertib for patients with castration-resistant and neuroendocrine prostate cancer: efficacy and biomarkers. Clin. Cancer Res. 25, 43–51 (2019).
Gandaglia, G. et al. Distribution of metastatic sites in patients with prostate cancer: a population-based analysis. Prostate 74, 210–216 (2014).
Haffner, M. C. et al. Tracking the clonal origin of lethal prostate cancer. J. Clin. Invest. 123, 4918–4922 (2013).
van Dessel, L. F. et al. Application of circulating tumor DNA in prospective clinical oncology trials — standardization of preanalytical conditions. Mol. Oncol. 11, 295–304 (2017).
von Eyben, F. E., Picchio, M., von Eyben, R., Rhee, H. & Bauman, G. 68Ga-labeled prostate-specific membrane antigen ligand positron emission tomography/computed tomography for prostate cancer: a systematic review and meta-analysis. Eur. Urol. Focus. 4, 686–693 (2018).
Damjanovic, J. et al. 68Ga-PSMA-PET/CT for the evaluation of liver metastases in patients with prostate cancer. Cancer Imaging 19, 37 (2019).
Roviello, G., Petrioli, R., Villari, D. & D’Angelo, A. Treating de novo metastatic castration-sensitive prostate cancer with visceral metastases: an evolving issue. Clin. Genitourin. Cancer 19, 83–86 (2021).
Khreish, F. et al. Response and outcome of liver metastases in patients with metastatic castration-resistant prostate cancer (mCRPC) undergoing 177Lu-PSMA-617 radioligand therapy. Eur. J. Nucl. Med. Mol. Imaging 48, 103–112 (2021).
Bubendorf, L. et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum. Pathol. 31, 578–583 (2000).
Shah, R. B. et al. Androgen-independent prostate cancer is a heterogeneous group of diseases: lessons from a rapid autopsy program. Cancer Res. 64, 9209–9216 (2004).
Pezaro, C. et al. Visceral disease in castration-resistant prostate cancer. Eur. Urol. 65, 270–273 (2014).
van den Bergh, G. P. A. et al. Incidence and survival of castration-resistant prostate cancer patients with visceral metastases: results from the Dutch CAPRI-registry. Prostate Cancer Prostatic Dis. 26, 162–169 (2023).
Deng, Y. et al. A surveillance, epidemiology and end results database analysis of the prognostic value of organ-specific metastases in patients with advanced prostatic adenocarcinoma. Oncol. Lett. 18, 1057–1070 (2019).
Shou, J., Zhang, Q., Wang, S. & Zhang, D. The prognosis of different distant metastases pattern in prostate cancer: a population based retrospective study. Prostate 78, 491–497 (2018).
Cotogno, P. M., Ranasinghe, L. K., Ledet, E. M., Lewis, B. E. & Sartor, O. Laboratory-based biomarkers and liver metastases in metastatic castration-resistant prostate cancer. Oncologist 23, 791–797 (2018).
Ranasinghe, L. et al. Relationship between serum markers and volume of liver metastases in castration-resistant prostate cancer. Cancer Treat. Res. Commun. 20, 100151 (2019).
Rahbar, K. et al. German multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J. Nucl. Med. 58, 85–90 (2017).
Ahmadzadehfar, H. et al. Prior therapies as prognostic factors of overall survival in metastatic castration-resistant prostate cancer patients treated with [177Lu]Lu-PSMA-617. A WARMTH multicenter study (the 617 trial). Eur. J. Nucl. Med. Mol. Imaging 48, 113–122 (2021).
Kessel, K. et al. Second line chemotherapy and visceral metastases are associated with poor survival in patients with mCRPC receiving 177Lu-PSMA-617. Theranostics 9, 4841–4848 (2019).
Luna-Gutierrez, M. et al. Improving overall survival and quality of life in patients with prostate cancer and neuroendocrine tumors using 177Lu-iPSMA and 177Lu-DOTATOC: experience after 905 treatment doses. Pharmaceutics 15, 1988 (2023).
Fendler, W. P. et al. Prostate-specific membrane antigen ligand positron emission tomography in men with nonmetastatic castration-resistant prostate cancer. Clin. Cancer Res. 25, 7448–7454 (2019).
Hofman, M. S. et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet 395, 1208–1216 (2020).
Maitland, N. J. Resistance to antiandrogens in prostate cancer: is it inevitable, intrinsic or induced? Cancers 13, 327 (2021).
Buxton, A. K., Abbasova, S., Bevan, C. L. & Leach, D. A. Liver microenvironment response to prostate cancer metastasis and hormonal therapy. Cancers 14, 6189 (2022).
Berry, W. R., Laszlo, J., Cox, E., Walker, A. & Paulson, D. Prognostic factors in metastatic and hormonally unresponsive carcinoma of the prostate. Cancer 44, 763–775 (1979).
Factors in the prognosis of carcinoma of the prostate: a cooperative study. The Veterans Administration Cooperative Urological Research Group. J. Urol. 100, 59–65 (1968).
Petrylak, D. P., Scher, H. I., Li, Z., Myers, C. E. & Geller, N. L. Prognostic factors for survival of patients with bidimensionally measurable metastatic hormone-refractory prostatic cancer treated with single-agent chemotherapy. Cancer 70, 2870–2878 (1992).
Chi, K. N. et al. A prognostic index model for predicting overall survival in patients with metastatic castration-resistant prostate cancer treated with abiraterone acetate after docetaxel. Ann. Oncol. 27, 454–460 (2016).
Conteduca, V. et al. Impact of visceral metastases on outcome to abiraterone after docetaxel in castration-resistant prostate cancer patients. Future Oncol. 11, 2881–2891 (2015).
Vasil’eva, N. N. & Milievskaia, I. L. [Neurogenic stomach neoplasms induced by nitrosomethylures in Syrian hamsters]. Arkh Patol. 39, 66–71 (1977).
Armstrong, A. J. et al. A contemporary prognostic nomogram for men with hormone-refractory metastatic prostate cancer: a TAX327 study analysis. Clin. Cancer Res. 13, 6396–6403 (2007).
Loriot, Y. et al. Enzalutamide in castration-resistant prostate cancer patients with visceral disease in the liver and/or lung: outcomes from the randomized controlled phase 3 AFFIRM trial. Cancer 123, 253–262 (2017).
Alumkal, J. J. et al. Effect of visceral disease site on outcomes in patients with metastatic castration-resistant prostate cancer treated with enzalutamide in the PREVAIL trial. Clin. Genitourin. Cancer 15, 610–617 e613 (2017).
Beer, T. M. et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl. J. Med. 371, 424–433 (2014).
Baciarello, G. et al. Impact of abiraterone acetate plus prednisone in patients with castration-sensitive prostate cancer and visceral metastases over four years of follow-up: a post-hoc exploratory analysis of the LATITUDE study. Eur. J. Cancer 162, 56–64 (2022).
Satapathy, S., Mittal, B. R. & Sood, A. Visceral metastases as predictors of response and survival outcomes in patients of castration-resistant prostate cancer treated with 177Lu-labeled prostate-specific membrane antigen radioligand therapy: a systematic review and meta-analysis. Clin. Nucl. Med. 45, 935–942 (2020).
Armstrong, A. J. et al. Development and validation of a prognostic model for overall survival in chemotherapy-naive men with metastatic castration-resistant prostate cancer. Ann. Oncol. 29, 2200–2207 (2018).
Akamatsu, S. et al. Development and validation of a novel prognostic model for predicting overall survival in treatment-naive castration-sensitive metastatic prostate cancer. Eur. Urol. Oncol. 2, 320–328 (2019).
Gafita, A. et al. Nomograms to predict outcomes after 177Lu-PSMA therapy in men with metastatic castration-resistant prostate cancer: an international, multicentre, retrospective study. Lancet Oncol. 22, 1115–1125 (2021).
Labrecque, M. P. et al. Molecular profiling stratifies diverse phenotypes of treatment-refractory metastatic castration-resistant prostate cancer. J. Clin. Invest. 129, 4492–4505 (2019).
Aggarwal, R. et al. Clinical and genomic characterization of treatment-emergent small-cell neuroendocrine prostate cancer: a multi-institutional prospective study. J. Clin. Oncol. 36, 2492–2503 (2018).
Bakht, M. K. et al. Landscape of prostate-specific membrane antigen heterogeneity and regulation in AR-positive and AR-negative metastatic prostate cancer. Nat. Cancer 4, 699–715 (2023).
Feng, E. et al. Intrinsic molecular subtypes of metastatic castration-resistant prostate cancer. Clin. Cancer Res. 28, 5396–5404 (2022).
Conteduca, V. et al. Clinical features of neuroendocrine prostate cancer. Eur. J. Cancer 121, 7–18 (2019).
Thysell, E. et al. Gene expression profiles define molecular subtypes of prostate cancer bone metastases with different outcomes and morphology traceable back to the primary tumor. Mol. Oncol. 13, 1763–1777 (2019).
Ku, S. Y., Gleave, M. E. & Beltran, H. Towards precision oncology in advanced prostate cancer. Nat. Rev. Urol. 16, 645–654 (2019).
Paschalis, A. et al. Prostate-specific membrane antigen heterogeneity and DNA repair defects in prostate cancer. Eur. Urol. 76, 469–478 (2019).
Kumar, A. et al. Substantial interindividual and limited intraindividual genomic diversity among tumors from men with metastatic prostate cancer. Nat. Med. 22, 369–378 (2016).
Alshalalfa, M. et al. Clinicogenomic characterization of prostate cancer liver metastases. Prostate Cancer Prostatic Dis. 25, 366–369 (2022).
Samarzija, I. Site-specific and common prostate cancer metastasis genes as suggested by meta-analysis of gene expression data. Life 11, 636 (2021).
Faltermeier, C. M. et al. Functional screen identifies kinases driving prostate cancer visceral and bone metastasis. Proc. Natl Acad. Sci. USA 113, E172–E181 (2016).
Lee, J. W. et al. Hepatocytes direct the formation of a pro-metastatic niche in the liver. Nature 567, 249–252 (2019).
Eveno, C. et al. Proof of prometastatic niche induction by hepatic stellate cells. J. Surg. Res. 194, 496–504 (2015).
Kopp, W. [Morphometric distribution of HLA-DR-coded immunocytes in gingiva biopsy specimens with periodontal disease]. Dtsch. Zahnarztl. Z. 45, 93–97 (1990).
Kondo, T. et al. The impact of hepatic fibrosis on the incidence of liver metastasis from colorectal cancer. Br. J. Cancer 115, 34–39 (2016).
Ohlund, D., Elyada, E. & Tuveson, D. Fibroblast heterogeneity in the cancer wound. J. Exp. Med. 211, 1503–1523 (2014).
Costa-Silva, B. et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 17, 816–826 (2015).
Hintz, H. M. et al. Imaging fibroblast activation protein alpha improves diagnosis of metastatic prostate cancer with positron emission tomography. Clin. Cancer Res. 26, 4882–4891 (2020).
James, N. D. et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N. Engl. J. Med. 377, 338–351 (2017).
Colomba, E. et al. Liver tests increase on abiraterone acetate in men with metastatic prostate cancer: natural history, management and outcome. Eur. J. Cancer 129, 117–122 (2020).
Yun, G. Y. et al. Atypical onset of bicalutamide-induced liver injury. World J. Gastroenterol. 22, 4062–4065 (2016).
Kim, O. H. et al. Fluid shear stress facilitates prostate cancer metastasis through Piezo1-Src-YAP axis. Life Sci. 308, 120936 (2022).
Akoto, T. & Saini, S. Role of exosomes in prostate cancer metastasis. Int. J. Mol. Sci. 22, 3528 (2021).
Vagner, T. et al. Large extracellular vesicles carry most of the tumour DNA circulating in prostate cancer patient plasma. J. Extracell. Vesicles 7, 1505403 (2018).
Teng, Y. et al. MVP-mediated exosomal sorting of miR-193a promotes colon cancer progression. Nat. Commun. 8, 14448 (2017).
Li, S. et al. Tumour-derived exosomes in liver metastasis: a Pandora’s box. Cell Prolif. 56, e13452 (2023).
Hoshino, A. et al. Tumour exosome integrins determine organotropic metastasis. Nature 527, 329–335 (2015).
Li, C. et al. hnRNPA2B1-mediated extracellular vesicles sorting of miR-122-5p potentially promotes lung cancer progression. Int. Mol. J. Sci. 22, 12866 (2021).
Zhang, C. et al. Cancer-derived exosomal HSPC111 promotes colorectal cancer liver metastasis by reprogramming lipid metabolism in cancer-associated fibroblasts. Cell Death Dis. 13, 57 (2022).
Zeng, Z. et al. Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat. Commun. 9, 5395 (2018).
Kim, J. et al. Three-dimensional human liver-chip emulating premetastatic niche formation by breast cancer-derived extracellular vesicles. ACS Nano 14, 14971–14988 (2020).
Novizio, N. et al. ANXA1 contained in EVs regulates macrophage polarization in tumor microenvironment and promotes pancreatic cancer progression and metastasis. Int. J. Mol. Sci. 22, 11018 (2021).
Baig, M. S. et al. Tumor-derived exosomes in the regulation of macrophage polarization. Inflamm. Res. 69, 435–451 (2020).
Chang, Y. T. et al. Pancreatic cancer-derived small extracellular vesical Ezrin regulates macrophage polarization and promotes metastasis. Am. J. Cancer Res. 10, 12–37 (2020).
Yin, X. et al. MiR-26b-5p in small extracellular vesicles derived from dying tumor cells after irradiation enhances the metastasis promoting microenvironment in esophageal squamous cell carcinoma. Cancer Lett. 541, 215746 (2022).
Sun, H. et al. Hypoxia-inducible exosomes facilitate liver-tropic premetastatic niche in colorectal cancer. Hepatology 74, 2633–2651 (2021).
Kmiec, Z. Cooperation of liver cells in health and disease. Adv. Anat. Embryol. Cell Biol. 161, 1–151 (2001). III-XIII.
Matsumura, H. et al. Kupffer cells decrease metastasis of colon cancer cells to the liver in the early stage. Int. J. Oncol. 45, 2303–2310 (2014).
Condamine, T. & Gabrilovich, D. I. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 32, 19–25 (2011).
Zhao, W. et al. Hepatic stellate cells promote tumor progression by enhancement of immunosuppressive cells in an orthotopic liver tumor mouse model. Lab. Invest. 94, 182–191 (2014).
Bu, P. et al. Aldolase B-mediated fructose metabolism drives metabolic reprogramming of colon cancer liver metastasis. Cell Metab. 27, 1249–1262 e1244 (2018).
Spratt, D. E. et al. Utility of FDG-PET in clinical neuroendocrine prostate cancer. Prostate 74, 1153–1159 (2014).
Yates, C. C., Shepard, C. R., Stolz, D. B. & Wells, A. Co-culturing human prostate carcinoma cells with hepatocytes leads to increased expression of E-cadherin. Br. J. Cancer 96, 1246–1252 (2007).
Wells, A., Yates, C. & Shepard, C. R. E-cadherin as an indicator of mesenchymal to epithelial reverting transitions during the metastatic seeding of disseminated carcinomas. Clin. Exp. Metastasis 25, 621–628 (2008).
Ma, B. & Wells, A. The mitogen-activated protein (MAP) kinases p38 and extracellular signal-regulated kinase (ERK) are involved in hepatocyte-mediated phenotypic switching in prostate cancer cells. J. Biol. Chem. 289, 11153–11161 (2014).
Ma, B. et al. Liver protects metastatic prostate cancer from induced death by activating E-cadherin signaling. Hepatology 64, 1725–1742 (2016).
Ma, B., Khazali, A., Shao, H., Jiang, Y. & Wells, A. Expression of E-cadherin and specific CXCR3 isoforms impact each other in prostate cancer. Cell Commun. Signal. 17, 164 (2019).
Lin, H. Y. et al. Matriptase-2/NR4A3 axis switches TGF-β action toward suppression of prostate cancer cell invasion, tumor growth, and metastasis. Oncogene 41, 2833–2845 (2022).
Wu, Q., Dhir, R. & Wells, A. Altered CXCR3 isoform expression regulates prostate cancer cell migration and invasion. Mol. Cancer 11, 3 (2012).
Zhu, W. B., Zhao, Z. F. & Zhou, X. AMD3100 inhibits epithelial-mesenchymal transition, cell invasion, and metastasis in the liver and the lung through blocking the SDF-1ɑ/CXCR4 signaling pathway in prostate cancer. J. Cell Physiol. 234, 11746–11759 (2019).
Steele, R., Mott, J. L. & Ray, R. B. MBP-1 upregulates miR-29b that represses Mcl-1, collagens, and matrix-metalloproteinase-2 in prostate cancer cells. Genes. Cancer 1, 381–387 (2010).
Ru, P. et al. miRNA-29b suppresses prostate cancer metastasis by regulating epithelial-mesenchymal transition signaling. Mol. Cancer Ther. 11, 1166–1173 (2012).
Liu, C. et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat. Med. 17, 211–215 (2011).
Tauriello, D. V. F. et al. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 554, 538–543 (2018).
Gao, Y. et al. Tumor immunoevasion by the conversion of effector NK cells into type 1 innate lymphoid cells. Nat. Immunol. 18, 1004–1015 (2017).
Correia, A. L. et al. Hepatic stellate cells suppress NK cell-sustained breast cancer dormancy. Nature 594, 566–571 (2021).
Ducimetiere, L. et al. Conventional NK cells and tissue-resident ILC1s join forces to control liver metastasis. Proc. Natl Acad. Sci. USA 118, e2026271118 (2021).
Donkor, M. K. et al. T cell surveillance of oncogene-induced prostate cancer is impeded by T cell-derived TGF-β1 cytokine. Immunity 35, 123–134 (2011).
Yu, J. et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat. Med. 27, 152–164 (2021).
Ham, B. et al. TNF receptor-2 facilitates an immunosuppressive microenvironment in the liver to promote the colonization and growth of hepatic metastases. Cancer Res. 75, 5235–5247 (2015).
Yang, S. et al. Integrated multi-omics landscape of liver metastases. Gastroenterology 164, 407–423 e417 (2023).
Wu, J. B. et al. MAOA-dependent activation of Shh-IL6-RANKL signaling network promotes prostate cancer metastasis by engaging tumor-stromal cell interactions. Cancer Cell 31, 368–382 (2017).
Wegiel, B. et al. Multiple cellular mechanisms related to cyclin A1 in prostate cancer invasion and metastasis. J. Natl Cancer Inst. 100, 1022–1036 (2008).
Klezovitch, O. et al. Hepsin promotes prostate cancer progression and metastasis. Cancer Cell 6, 185–195 (2004).
Hsin, F., Hsu, Y. C., Tsai, Y. F., Lin, S. W. & Liu, H. M. The transmembrane serine protease hepsin suppresses type I interferon induction by cleaving STING. Sci. Signal 14, eabb4752 (2021).
Su, W. et al. The polycomb repressor complex 1 drives double-negative prostate cancer metastasis by coordinating stemness and immune suppression. Cancer Cell 36, 139–155 e110 (2019).
Sailer, V. et al. Experimental in vitro, ex vivo and in vivo models in prostate cancer research. Nat. Rev. Urol. 20, 158–178 (2023).
Gao, D. et al. Organoid cultures derived from patients with advanced prostate cancer. Cell 159, 176–187 (2014).
Pauli, C. et al. Personalized in vitro and in vivo cancer models to guide precision medicine. Cancer Discov. 7, 462–477 (2017).
Hsiao, A. Y. et al. Microfluidic system for formation of PC-3 prostate cancer co-culture spheroids. Biomaterials 30, 3020–3027 (2009).
Bock, N. et al. In vitro engineering of a bone metastases model allows for study of the effects of antiandrogen therapies in advanced prostate cancer. Sci. Adv. 7, eabg2564 (2021).
Lau, W. M. et al. Identification of prospective factors promoting osteotropism in breast cancer: a potential role for CITED2. Int. J. Cancer 126, 876–884 (2010).
Qi, X. et al. An oncogenic hepatocyte-induced orthotopic mouse model of hepatocellular cancer arising in the setting of hepatic inflammation and fibrosis. J. Vis. Exp. https://doi.org/10.3791/59368 (2019).
Liu, K. et al. A novel mouse model for liver metastasis of prostate cancer reveals dynamic tumour-immune cell communication. Cell Prolif. 54, e13056 (2021).
Acevedo, V. D. et al. Inducible FGFR-1 activation leads to irreversible prostate adenocarcinoma and an epithelial-to-mesenchymal transition. Cancer Cell 12, 559–571 (2007).
Cho, H. et al. RapidCaP, a novel GEM model for metastatic prostate cancer analysis and therapy, reveals myc as a driver of Pten-mutant metastasis. Cancer Discov. 4, 318–333 (2014).
Taranda, J. et al. Combined whole-organ imaging at single-cell resolution and immunohistochemical analysis of prostate cancer and its liver and brain metastases. Cell Rep. 37, 110027 (2021).
Zaporowska-Stachowiak, I. et al. Managing metastatic bone pain: new perspectives, different solutions. Biomed. Pharmacother. 93, 1277–1284 (2017).
Alderton, G. K. Metastasis: directions to metastatic sites. Nat. Rev. Cancer 15, 696–697 (2015).
Xu, N. et al. Risk factors of developing visceral metastases at diagnosis in prostate cancer patients. Transl. Cancer Res. 8, 928–938 (2019).
Steffens, J., Friedmann, W. & Lobeck, H. Immunohistochemical diagnosis of the metastasizing prostatic carcinoma. Eur. Urol. 11, 91–94 (1985).
Whitney, C. A. et al. In men with castration-resistant prostate cancer, visceral metastases predict shorter overall survival: what predicts visceral metastases? Results from the SEARCH database. Eur. Urol. Focus. 3, 480–486 (2017).
Minami, Y. & Kudo, M. Hepatic malignancies: correlation between sonographic findings and pathological features. World J. Radiol. 2, 249–256 (2010).
Sahani, D. V., Bajwa, M. A., Andrabi, Y., Bajpai, S. & Cusack, J. C. Current status of imaging and emerging techniques to evaluate liver metastases from colorectal carcinoma. Ann. Surg. 259, 861–872 (2014).
Tanaka, T. et al. Current imaging techniques for and imaging spectrum of prostate cancer recurrence and metastasis: a pictorial review. Radiographics 40, 709–726 (2020).
Namasivayam, S., Martin, D. R. & Saini, S. Imaging of liver metastases: MRI. Cancer Imaging 7, 2–9 (2007).
Niekel, M. C., Bipat, S. & Stoker, J. Diagnostic imaging of colorectal liver metastases with CT, MR imaging, FDG PET, and/or FDG PET/CT: a meta-analysis of prospective studies including patients who have not previously undergone treatment. Radiology 257, 674–684 (2010).
Floriani, I. et al. Performance of imaging modalities in diagnosis of liver metastases from colorectal cancer: a systematic review and meta-analysis. J. Magn. Reson. Imaging 31, 19–31 (2010).
Brimo, F. & Epstein, J. I. Immunohistochemical pitfalls in prostate pathology. Hum. Pathol. 43, 313–324 (2012).
de Galiza Barbosa, F. et al. Nonprostatic diseases on PSMA PET imaging: a spectrum of benign and malignant findings. Cancer Imaging 20, 23 (2020).
Jadvar, H. Molecular imaging of prostate cancer: PET radiotracers.AJR Am. J. Roentgenol. 199, 278–291 (2012).
Jadvar, H. Positron emission tomography in prostate cancer: summary of systematic reviews and meta-analysis. Tomography 1, 18–22 (2015).
Vaz, C. V. et al. Androgens enhance the glycolytic metabolism and lactate export in prostate cancer cells by modulating the expression of GLUT1, GLUT3, PFK, LDH and MCT4 genes. J. Cancer Res. Clin. Oncol. 142, 5–16 (2016).
Jadvar, H. Molecular imaging of prostate cancer with 18F-fluorodeoxyglucose PET. Nat. Rev. Urol. 6, 317–323 (2009).
Umbehr, M. H., Muntener, M., Hany, T., Sulser, T. & Bachmann, L. M. The role of 11C-choline and 18F-fluorocholine positron emission tomography (PET) and PET/CT in prostate cancer: a systematic review and meta-analysis. Eur. Urol. 64, 106–117 (2013).
Garcia, J. R. et al. [Low diagnostic yield of the 11C-choline PET/CT in the detection of liver metastasis from prostate cancer]. Rev. Esp. Med. Nucl. Imagen Mol. 33, 56–57 (2014).
Ghedini, P. et al. Liver metastases from prostate cancer at 11C-Choline PET/CT: a multicenter, retrospective analysis. Eur. J. Nucl. Med. Mol. Imaging 45, 751–758 (2018).
Bianchi, D., Rizzo, A., Bonacina, M., Zaniboni, A. & Savelli, G. Penile metastasis from prostate cancer detected by 18F-fluorocholine PET/CT. Clin. Nucl. Med. 46, e38–e39 (2021).
Dejust, S., Messaoud, L., Jallerat, P., Marical, V. & Morland, D. Hepatic metastases from prostatic adenocarcinoma without elevated 18F-choline activity. Clin. Nucl. Med. 43, 780–781 (2018).
Onner, H., Ozer, H., Celik, A. V., Yilmaz, F. & Kara Gedik, G. Isolated liver metastasis detected by 68Ga-PSMA PET/CT in newly diagnosed prostate cancer. Clin. Nucl. Med. 48, 259–260 (2023).
De Man, K. et al. 18F-PSMA-11 versus 68Ga-PSMA-11 positron emission tomography/computed tomography for staging and biochemical recurrence of prostate cancer: a prospective double-blind randomised cross-over trial. Eur. Urol. 82, 501–509 (2022).
Seniaray, N., Verma, R., Belho, E., Malik, D. & Mahajan, H. Diffuse pulmonary metastases from prostate cancer on 68Ga PSMA PET/CT. Clin. Nucl. Med. 44, 898–900 (2019).
Soydal, C., Ozkan, E., Yerlikaya, H., Utkan, G. & Kucuk, O. N. Widespread metastatic prostate carcinoma shown by 68Ga-PSMA PET/CT. Clin. Nucl. Med. 41, e294–e295 (2016).
Chan, M., Hsiao, E. & Turner, J. Cerebellar metastases from prostate cancer on 68Ga-PSMA PET/CT. Clin. Nucl. Med. 42, 193–194 (2017).
Seifert, R. et al. Second version of the prostate cancer molecular imaging standardized evaluation framework including response evaluation for clinical trials (PROMISE V2). Eur. Urol. 83, 405–412 (2023).
Watt, F. et al. A tissue-specific enhancer of the prostate-specific membrane antigen gene, FOLH1. Genomics 73, 243–254 (2001).
Noss, K. R., Wolfe, S. A. & Grimes, S. R. Upregulation of prostate specific membrane antigen/folate hydrolase transcription by an enhancer. Gene 285, 247–256 (2002).
Beltran, H. et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat. Med. 22, 298–305 (2016).
Bakht, M. K. et al. Neuroendocrine differentiation of prostate cancer leads to PSMA suppression. Endocr. Relat. Cancer 26, 131–146 (2018).
Thang, S. P. et al. Poor outcomes for patients with metastatic castration-resistant prostate cancer with low prostate-specific membrane antigen (PSMA) expression deemed ineligible for 177Lu-labelled PSMA radioligand therapy. Eur. Urol. Oncol. 2, 670–676 (2019).
Evans, M. J. et al. Noninvasive measurement of androgen receptor signaling with a positron-emitting radiopharmaceutical that targets prostate-specific membrane antigen. Proc. Natl Acad. Sci. USA 108, 9578–9582 (2011).
Shetty, D., Patel, D., Le, K., Bui, C. & Mansberg, R. Pitfalls in gallium-68 PSMA PET/CT interpretation-A pictorial review. Tomography 4, 182–193 (2018).
Ladron-de-Guevara, D., Canelo, A., Piottante, A. & Regonesi, C. False-positive 18F-prostate-specific membrane antigen-1007 PET/CT caused by hepatic multifocal inflammatory foci. Clin. Nucl. Med. 46, e80–e83 (2021).
Kesch, C. et al. High fibroblast-activation-protein expression in castration-resistant prostate cancer supports the use of FAPI-molecular theranostics. Eur. J. Nucl. Med. Mol. Imaging 49, 385–389 (2021).
Kessel, K. et al. Prostate-specific membrane antigen and fibroblast activation protein distribution in prostate cancer: preliminary data on immunohistochemistry and PET imaging. Ann. Nucl. Med. 36, 293–301 (2022).
Bluemel, C. et al. 68Ga-PSMA-PET/CT in patients with biochemical prostate cancer recurrence and negative 18F-Choline-PET/CT. Clin. Nucl. Med. 41, 515–521 (2016).
Bakht, M. K. et al. Differential expression of glucose transporters and hexokinases in prostate cancer with a neuroendocrine gene signature: a mechanistic perspective for 18F-FDG imaging of PSMA-suppressed tumors. J. Nucl. Med. 61, 904–910 (2020).
Jadvar, H. The VISION forward: recognition and implication of PSMA-/18F-FDG+ mCRPC. J. Nucl. Med. 63, 812–815 (2022).
Ignatiadis, M., Sledge, G. W. & Jeffrey, S. S. Liquid biopsy enters the clinic - implementation issues and future challenges. Nat. Rev. Clin. Oncol. 18, 297–312 (2021).
Xu, L. et al. The novel association of circulating tumor cells and circulating megakaryocytes with prostate cancer prognosis. Clin. Cancer Res. 23, 5112–5122 (2017).
Aceto, N. et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 158, 1110–1122 (2014).
Hille, C. & Pantel, K. Prostate cancer: circulating tumour cells in prostate cancer. Nat. Rev. Urol. 15, 265–266 (2018).
Chen, J. F. et al. Subclassification of prostate cancer circulating tumor cells by nuclear size reveals very small nuclear circulating tumor cells in patients with visceral metastases. Cancer 121, 3240–3251 (2015).
Snyder, M. W., Kircher, M., Hill, A. J., Daza, R. M. & Shendure, J. Cell-free DNA comprises an in vivo nucleosome footprint that informs its tissues-of-origin. Cell 164, 57–68 (2016).
Mouliere, F. et al. Enhanced detection of circulating tumor DNA by fragment size analysis. Sci. Transl. Med. 10, eaat4921 (2018).
Mehra, N. et al. Plasma cell-free DNA concentration and outcomes from taxane therapy in metastatic castration-resistant prostate cancer from two phase III trials (FIRSTANA and PROSELICA). Eur. Urol. 74, 283–291 (2018).
Vandekerkhove, G. et al. Circulating tumor DNA abundance and potential utility in de novo metastatic prostate cancer. Eur. Urol. 75, 667–675 (2019).
Wyatt, A. W. et al. Concordance of circulating tumor DNA and matched metastatic tissue biopsy in prostate cancer. J. Natl Cancer Inst. 109, djx118 (2017).
Schweizer, M. T. et al. Concordance of DNA repair gene mutations in paired primary prostate cancer samples and metastatic tissue or cell-free DNA. JAMA Oncol. 7, 1–5 (2021).
Beltran, H. et al. Circulating tumor DNA profile recognizes transformation to castration-resistant neuroendocrine prostate cancer. J. Clin. Invest. 130, 1653–1668 (2020).
Del Re, M. et al. The detection of androgen receptor splice variant 7 in plasma-derived exosomal RNA strongly predicts resistance to hormonal therapy in metastatic prostate cancer patients. Eur. Urol. 71, 680–687 (2017).
Zhang, Y. et al. Loss of exosomal miR-146a-5p from cancer-associated fibroblasts after androgen deprivation therapy contributes to prostate cancer metastasis. J. Exp. Clin. Cancer Res. 39, 282 (2020).
Bhagirath, D. et al. microRNA-1246 is an exosomal biomarker for aggressive prostate cancer. Cancer Res. 78, 1833–1844 (2018).
Davis, I. D. et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N. Engl. J. Med. 381, 121–131 (2019).
Goodman, O. B. Jr et al. Exploratory analysis of the visceral disease subgroup in a phase III study of abiraterone acetate in metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 17, 34–39 (2014).
Berthold, D. R. et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J. Clin. Oncol. 26, 242–245 (2008).
de Wit, R. et al. Cabazitaxel versus abiraterone or enzalutamide in metastatic prostate cancer. N. Engl. J. Med. 381, 2506–2518 (2019).
Alabi, B. R., Liu, S. & Stoyanova, T. Current and emerging therapies for neuroendocrine prostate cancer. Pharmacol. Ther. 238, 108255 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04709276 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05413421 (2024).
Fizazi, K. et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): a multicentre, open-label, randomised, phase 3 study with a 2 x 2 factorial design. Lancet 399, 1695–1707 (2022).
Hussain, M. et al. Darolutamide plus androgen-deprivation therapy and docetaxel in metastatic hormone-sensitive prostate cancer by disease volume and risk subgroups in the phase III ARASENS trial. J. Clin. Oncol. 41, 3595–3607 (2023).
Clarke, N. W. Abiraterone and olaparib for metastatic castration-resistant prostate cancer. N. Engl. J. Med. Evid. 1, 9 (2022).
Agarwal, N. et al. Talazoparib plus enzalutamide in men with first-line metastatic castration-resistant prostate cancer (TALAPRO-2): a randomised, placebo-controlled, phase 3 trial. Lancet 402, 291–303 (2023).
de Bono, J. S. et al. Talazoparib monotherapy in metastatic castration-resistant prostate cancer with DNA repair alterations (TALAPRO-1): an open-label, phase 2 trial. Lancet Oncol. 22, 1250–1264 (2021).
Chi, K. N. et al. Niraparib plus abiraterone acetate with prednisone in patients with metastatic castration-resistant prostate cancer and homologous recombination repair gene alterations: second interim analysis of the randomized phase III MAGNITUDE trial. Ann. Oncol. 34, 772–782 (2023).
Abida, W. et al. Rucaparib in men with metastatic castration-resistant prostate cancer harboring a BRCA1 or BRCA2 gene alteration. J. Clin. Oncol. 38, 3763–3772 (2020).
Petrylak, D. P. et al. Pembrolizumab plus docetaxel for patients with metastatic castration-resistant prostate cancer (mCRPC): randomized, double-blind, phase 3 KEYNOTE-921 study. J. Clin. Oncol. 41, 19–19 (2023).
Hennrich, U. & Eder, M. [177Lu]Lu-PSMA-617 (PluvictoTM): the first FDA-approved radiotherapeutical for treatment of prostate cancer. Pharmaceuticals 15, 1292 (2022).
Sartor, O. et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 385, 1091–1103 (2021).
Pouessel, D. et al. Liver metastases in prostate carcinoma: clinical characteristics and outcome. BJU Int. 99, 807–811 (2007).
Ferdinandus, J. et al. Predictors of response to radioligand therapy of metastatic castrate-resistant prostate cancer with 177Lu-PSMA-617. J. Nucl. Med. 58, 312–319 (2017).
Violet, J. et al. Dosimetry of 177Lu-PSMA-617 in metastatic castration-resistant prostate cancer: correlations between pretherapeutic imaging and whole-body tumor dosimetry with treatment outcomes. J. Nucl. Med. 60, 517–523 (2019).
Park, I. K. et al. Effect of equine-assisted activities on cardiac autonomic function in children with cerebral palsy: a pilot randomized-controlled trial. J. Altern. Complement. Med. 27, 96–102 (2021).
Kyte, J. A. Cancer vaccination with telomerase peptide GV1001. Expert. Opin. Investig. Drugs 18, 687–694 (2009).
Kim, J. W. et al. Anti-metastatic effect of GV1001 on prostate cancer cells; roles of GnRHR-mediated Gɑs-cAMP pathway and AR-YAP1 axis. Cell Biosci. 11, 191 (2021).
Agarwal, N. et al. Cabozantinib in combination with atezolizumab in patients with metastatic castration-resistant prostate cancer: results from an expansion cohort of a multicentre, open-label, phase 1b trial (COSMIC-021). Lancet Oncol. 23, 899–909 (2022).
Agarwal, N. et al. A phase III, randomized, open-label study (CONTACT-02) of cabozantinib plus atezolizumab versus second novel hormone therapy in patients with metastatic castration-resistant prostate cancer. Future Oncol. 18, 1185–1198 (2022).
Pang, Y. et al. Development of FAPI tetramers to improve tumor uptake and efficacy of FAPI radioligand therapy. J. Nucl. Med. 64, 1449–1455 (2023).
Feuerecker, B. et al. Activity and adverse events of actinium-225-PSMA-617 in advanced metastatic castration-resistant prostate cancer after failure of lutetium-177-PSMA. Eur. Urol. 79, 343–350 (2021).
Ballal, S. et al. Long-term survival outcomes of salvage [225Ac]Ac-PSMA-617 targeted alpha therapy in patients with PSMA-expressing end-stage metastatic castration-resistant prostate cancer: a real-world study. Eur. J. Nucl. Med. Mol. Imaging 50, 3777–3789 (2023).
Sathekge, M. M., Bruchertseifer, F., Vorster, M., Morgenstern, A. & Lawal, I. O. Global experience with PSMA-based alpha therapy in prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 49, 30–46 (2021).
Zang, J. et al. First-in-human study of 177Lu-EB-PSMA-617 in patients with metastatic castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 46, 148–158 (2019).
Wang, G. et al. A single-arm, low-dose, prospective study of 177Lu-EB-PSMA radioligand therapy in patients with metastatic castration-resistant prostate cancer. J. Nucl. Med. 64, 611–617 (2023).
Ost, P. et al. Metastasis-directed therapy of regional and distant recurrences after curative treatment of prostate cancer: a systematic review of the literature. Eur. Urol. 67, 852–863 (2015).
Wang, S. C., McCarthy, L. P. & Mehdi, S. Isolated hepatic metastasis from prostate carcinoma. Urol. Case Rep. 10, 51–53 (2017).
Battaglia, A. et al. Metastasectomy for visceral and skeletal oligorecurrent prostate cancer. World J. Urol. 37, 1543–1549 (2019).
Zager, J. S. et al. FOCUS phase 3 trial results: percutaneous hepatic perfusion (PHP) with melphalan for patients with ocular melanoma liver metastases (PHP-OCM-301/301A). J. Clin. Oncol. 40, 9510–9510 (2022).
Chalkidou, A. et al. Stereotactic ablative body radiotherapy in patients with oligometastatic cancers: a prospective, registry-based, single-arm, observational, evaluation study. Lancet Oncol. 22, 98–106 (2021).
Wang, H., Li, X., Peng, R., Wang, Y. & Wang, J. Stereotactic ablative radiotherapy for colorectal cancer liver metastasis. Semin. Cancer Biol. 71, 21–32 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT02239900 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT02710253 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT02843165 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT02888743 (2024).
Corn, P. G. et al. Cabazitaxel plus carboplatin for the treatment of men with metastatic castration-resistant prostate cancers: a randomised, open-label, phase 1–2 trial. Lancet Oncol. 20, 1432–1443 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04592237 (2024).
Blum, A., Wang, P. & Zenklusen, J. C. SnapShot: TCGA analyzed tumors. Cell 173, 530 (2018).
Cerami, E. et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2, 401–404 (2012).
Robinson, D. et al. Integrative clinical genomics of advanced prostate cancer. Cell 161, 1215–1228 (2015).
Fizazi, K. et al. Health-related quality of life and pain outcomes with [177Lu]Lu-PSMA-617 plus standard of care versus standard of care in patients with metastatic castration-resistant prostate cancer (VISION): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 24, 597–610 (2023).
Chi, K. N. et al. Niraparib and abiraterone acetate for metastatic castration-resistant prostate cancer. J. Clin. Oncol. 41, 3339–3351 (2023).
Kelly, W. K. et al. Randomized, double-blind, placebo-controlled phase III trial comparing docetaxel and prednisone with or without bevacizumab in men with metastatic castration-resistant prostate cancer: CALGB 90401. J. Clin. Oncol. 30, 1534–1540 (2012).
Chi, K. N. et al. Custirsen in combination with docetaxel and prednisone for patients with metastatic castration-resistant prostate cancer (SYNERGY trial): a phase 3, multicentre, open-label, randomised trial. Lancet Oncol. 18, 473–485 (2017).
Saad, F. et al. Orteronel plus prednisone in patients with chemotherapy-naive metastatic castration-resistant prostate cancer (ELM-PC 4): a double-blind, multicentre, phase 3, randomised, placebo-controlled trial. Lancet Oncol. 16, 338–348 (2015).
Oudard, S. et al. Cabazitaxel versus docetaxel as first-line therapy for patients with metastatic castration-resistant prostate cancer: a randomized phase III trial-FIRSTANA. J. Clin. Oncol. 35, 3189–3197 (2017).
Vogelzang, N. J. et al. Efficacy and safety of autologous dendritic cell-based immunotherapy, docetaxel, and prednisone vs placebo in patients with metastatic castration-resistant prostate cancer: the VIABLE phase 3 randomized clinical trial. JAMA Oncol. 8, 546–552 (2022).
Saad, F. et al. Apalutamide plus abiraterone acetate and prednisone versus placebo plus abiraterone and prednisone in metastatic, castration-resistant prostate cancer (ACIS): a randomised, placebo-controlled, double-blind, multinational, phase 3 study. Lancet Oncol. 22, 1541–1559 (2021).
Morris, M. J. et al. Randomized phase III study of enzalutamide compared with enzalutamide plus abiraterone for metastatic castration-resistant prostate cancer (Alliance A031201 Trial). J. Clin. Oncol. 41, 3352–3362 (2023).
Kellokumpu-Lehtinen, P. L. et al. 2-Weekly versus 3-weekly docetaxel to treat castration-resistant advanced prostate cancer: a randomised, phase 3 trial. Lancet Oncol. 14, 117–124 (2013).
Beer, T. M. et al. Custirsen (OGX-011) combined with cabazitaxel and prednisone versus cabazitaxel and prednisone alone in patients with metastatic castration-resistant prostate cancer previously treated with docetaxel (AFFINITY): a randomised, open-label, international, phase 3 trial. Lancet Oncol. 18, 1532–1542 (2017).
Fizazi, K. et al. Phase III, randomized, double-blind, multicenter trial comparing orteronel (TAK-700) plus prednisone with placebo plus prednisone in patients with metastatic castration-resistant prostate cancer that has progressed during or after docetaxel-based therapy: ELM-PC 5. J. Clin. Oncol. 33, 723–731 (2015).
Eisenberger, M. et al. Phase III study comparing a reduced dose of cabazitaxel (20 mg/m2) and the currently approved dose (25 mg/m2) in postdocetaxel patients with metastatic castration-resistant prostate cancer-PROSELICA. J. Clin. Oncol. 35, 3198–3206 (2017).
Antonarakis, E. S. et al. Pembrolizumab plus olaparib for patients with previously treated and biomarker-unselected metastatic castration-resistant prostate cancer: the randomized, open-label, phase III KEYLYNK-010 trial. J. Clin. Oncol. 41, 3839–3850 (2023).
Smith, M. et al. Phase III study of cabozantinib in previously treated metastatic castration-resistant prostate cancer: COMET-1. J. Clin. Oncol. 34, 3005–3013 (2016).
Gravis, G. et al. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): a randomised, open-label, phase 3 trial. Lancet Oncol. 14, 149–158 (2013).
Chi, K. N. et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N. Engl. J. Med. 381, 13–24 (2019).
Agarwal, N. et al. Orteronel for metastatic hormone-sensitive prostate cancer: a multicenter, randomized, open-label phase III trial (SWOG-1216). J. Clin. Oncol. 40, 3301–3309 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT03179410 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT03582475 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT03910660 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT03896503 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04702737 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04848337 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04926181 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05582031 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05605522 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05652686 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05988918 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05691465 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT06094842 (2024).
Acknowledgements
This study was supported by Oriental Scholar Professorship, Shanghai Municipal Commission of Education, China; National Nature Science Foundation of China (82172621, 81972375); Shanghai Medical Innovation Research Special Project (21Y11904300); Shanghai Shenkang Research Physician Innovation and Transformation Ability Training Project (SHDC2022CRD035); General Program of Beijing Xisike Clinical Oncology Research Foundation (Y-MSDZD2021-0230, Y-2019AZMS-0012); China Urological Oncology Research Foundation, Primary Health Care Foundation, China; Chinese Anti-Cancer Association — Hengrui PARP Inhibitor Cancer Research Foundation; Shanghai Academic/Technology Research Leader (23XD1420600); Fudan University (FDUROP No.22065) and the Medical Science Data Center in Shanghai Medical College of Fudan University. The authors express their gratitude to all the patients and their families who have participated in the Fudan University Shanghai Cancer Center Prostate Cancer Liver Metastasis Registry (FUSCC-PCaLM) since 2020. They thank them for providing them with clinical research samples, imaging data and follow-up treatment information. Their invaluable contributions have greatly contributed to the understanding and exploration of prostate cancer liver metastasis for both clinicians and researchers.
Author information
Authors and Affiliations
Contributions
Y.Z., X.N., Y.W., X.L., J.P., B.F. and T.Z. researched data for the article. Y.Z., X.L., B.F. and T.Z. contributed substantially to discussion of the content. Y.Z., X.N., Y.W. wrote the article. Y.Z., Y.L. and D.Y. reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Urology thanks A. Kishan, A. Briganti and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ni, X., Wei, Y., Li, X. et al. From biology to the clinic — exploring liver metastasis in prostate cancer. Nat Rev Urol (2024). https://doi.org/10.1038/s41585-024-00875-x
Accepted:
Published:
DOI: https://doi.org/10.1038/s41585-024-00875-x