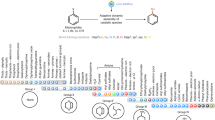
Abstract
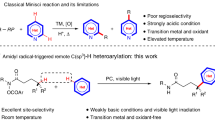
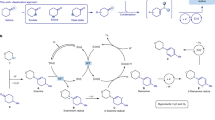
Over the past three decades, considerable progress has been made in the development of methods to construct sp2 carbon–nitrogen (C–N) bonds using palladium, copper or nickel catalysis1,2. However, the incorporation of alkyl substrates to form sp3 C–N bonds remains one of the major challenges in the field of cross-coupling chemistry. Here we demonstrate that the synergistic combination of copper catalysis and photoredox catalysis can provide a general platform from which to address this challenge. This cross-coupling system uses naturally abundant alkyl carboxylic acids and commercially available nitrogen nucleophiles as coupling partners. It is applicable to a wide variety of primary, secondary and tertiary alkyl carboxylic acids (through iodonium activation), as well as a vast array of nitrogen nucleophiles: nitrogen heterocycles, amides, sulfonamides and anilines can undergo C–N coupling to provide N-alkyl products in good to excellent efficiency, at room temperature and on short timescales (five minutes to one hour). We demonstrate that this C–N coupling protocol proceeds with high regioselectivity using substrates that contain several amine groups, and can also be applied to complex drug molecules, enabling the rapid construction of molecular complexity and the late-stage functionalization of bioactive pharmaceuticals.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ruiz-Castillo, P. & Buchwald, S. L. Applications of palladium-catalyzed C–N cross-coupling reactions. Chem. Rev. 116, 12564–12649 (2016).
Bhunia, S., Pawar, G. G., Kumar, S. V., Jiang, Y. & Ma, D. Selected copper-based reactions for C–N, C–O, C–S, and C–C bond formation. Angew. Chem. Int. Ed. 56, 16136–16179 (2017).
Knölker, H.-J. (ed.) The Alkaloids: Chemistry and Biology Vol. 70 (Elsevier, San Diego, 2011).
Vitaku, E., Smith, D. T. & Njardarson, J. T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 57, 10257–10274 (2014).
Ćirić-Marjanović, G. Recent advances in polyaniline research: polymerization mechanisms, structural aspects, properties and applications. Synth. Met. 177, 1–47 (2013).
Sambiagio, C., Marsden, S. P., Blacker, A. J. & McGowan, P. C. Copper catalysed Ullmann type chemistry: from mechanistic aspects to modern development. Chem. Soc. Rev. 43, 3525–3550 (2014).
Qiao, J. X. & Lam, P. Y. S. Copper-promoted carbon–heteroatom bond cross-coupling with boronic acids and derivatives. Synthesis 2011, 829–856 (2011).
Salvatore, R. N., Yoon, C. H. & Jung, K. W. Synthesis of secondary amines. Tetrahedron 57, 7785–7811 (2001).
Swamy, K. C. K., Kumar, N. N. B., Balaraman, E. & Kumar, K. V. P. P. Mitsunobu and related reactions: advances and applications. Chem. Rev. 109, 2551–2651 (2009).
Abdel-Magid, A. F. & Mehrman, S. J. A review on the use of sodium triacetoxyborohydride in the reductive amination of ketones and aldehydes. Org. Process Res. Dev. 10, 971–1031 (2006).
Huang, L., Arndt, M., Gooßen, K., Heydt, H. & Gooßen, L. J. Late transition metal-catalyzed hydroamination and hydroamidation. Chem. Rev. 115, 2596–2697 (2015).
Matier, C. D., Schwaben, J., Peters, J. C. & Fu, G. C. Copper-catalyzed alkylation of aliphatic amines induced by visible light. J. Am. Chem. Soc. 139, 17707–17710 (2017).
Peacock, D. M., Roos, C. B. & Hartwig, J. F. Palladium-catalyzed cross coupling of secondary and tertiary alkyl bromides with a nitrogen nucleophile. ACS Cent. Sci. 2, 647–652 (2016).
Curtius, T. 20. Hydrazide und azide organischer säuren I. Abhandlung. J. Prakt. Chem. 50, 275–294 (1894).
Shaw, M. H., Twilton, J. & MacMillan, D. W. C. Photoredox catalysis in organic chemistry. J. Org. Chem. 81, 6898–6926 (2016).
Twilton, J., Le, C., Zhang, P., Shaw, M. H., Evans, R. W. & MacMillan, D. W. C. The merger of transition metal and photocatalysis. Nat. Rev. Chem. 1, 0052 (2017).
Zuo, Z. et al. Merging photoredox with nickel catalysis: coupling of α-carboxyl sp3-carbons with aryl halides. Science 345, 437–440 (2014).
Zhang, X. & MacMillan, D. W. C. Alcohols as latent coupling fragments for metallaphotoredox catalysis: sp3–sp2 cross-coupling of oxalates with aryl halides. J. Am. Chem. Soc. 138, 13862–13865 (2016).
Zhao, W., Wurz, R. P., Peters, J. C. & Fu, G. C. Photoinduced, copper-catalyzed decarboxylative C–N coupling to generate protected amines: an alternative to the Curtius rearrangement. J. Am. Chem. Soc. 139, 12153–12156 (2017).
Mao, R., Frey, A., Balon, J. & Hu, X. Decarboxylative C(sp 3)–N cross-coupling via synergistic photoredox and copper catalysis. Nat. Catal. 1, 120–126 (2018).
Lowry, M. S. et al. Single-layer electroluminescent devices and photoinduced hydrogen production from an ionic iridium(III) complex. Chem. Mater. 17, 5712–5719 (2005).
Sanna, G., Pilo, M. I., Zoroddu, M. A. & Seeber, R. Electrochemical and spectroelectrochemical study of copper complexes with 1,10-phenanthrolines. Inorg. Chim. Acta 208, 153–158 (1993).
He, Z., Bae, M., Wu, J. & Jamison, T. F. Synthesis of highly functionalized polycyclic quinoxaline derivatives using visible-light photoredox catalysis. Angew. Chem. Int. Ed. 53, 14451–14455 (2014).
Minisci, F., Vismara, E., Fontana, F. & Barbosa, M. C. N. A new general method of homolytic alkylation of protonated heteroaromatic bases by carboxylic acids and iodosobenzene diacetate. Tetrahedr. Lett. 30, 4569–4572 (1989).
Tran, B. L., Li, B., Driess, M. & Hartwig, J. F. Copper-catalyzed intermolecular amidation and imidation of unactivated alkanes. J. Am. Chem. Soc. 136, 2555–2563 (2014).
Stepan, A. F. et al. Application of the bicyclo[1.1.1]pentane motif as a nonclassical phenyl ring bioisostere in the design of a potent and orally active γ-secretase inhibitor. J. Med. Chem. 55, 3414–3424 (2012).
Afagh, N. A. & Yudin, A. K. Chemoselectivity and the curious reactivity preferences of functional groups. Angew. Chem. Int. Ed. 49, 262–310 (2010).
Bordwell, F. G. Equilibrium acidities in dimethyl sulfoxide solution. Acc. Chem. Res. 21, 456–463 (1988).
Lõkov, M. et al. On the basicity of conjugated nitrogen heterocycles in different media. Eur. J. Org. Chem. 4475–4489 (2017).
Le, C. C. et al. A general small-scale reactor to enable standardization and acceleration of photocatalytic reactions. ACS Cent. Sci. 3, 647–653 (2017).
Acknowledgements
This research was supported by the National Institutes of Health National Institute of General Medical Sciences (R01 GM103558-03) and gifts from Merck, Bristol-Myers Squibb, Eli Lilly, Genentech and Johnson & Johnson. X.Z. is grateful for a postdoctoral fellowship from the Shanghai Institute of Organic Chemistry. We thank C. Liu and Y. Y. Loh for assistance in preparing this manuscript.
Reviewer information
Nature thanks S. Bagley and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
Y.L. and X.Z. performed and analysed the experiments. Y.L., X.Z. and D.W.C.M. designed the experiments. Y.L., X.Z. and D.W.C.M. prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Decarboxylative sp3 C–N couplings with a series of secondary alkyl acids.
An array of secondary alkyl carboxylic acids can be cross-coupled with 3-chloroindazole. The protocol provides the product as a single regioisomer in all cases. All yields are isolated. All reactions were replicated at least twice for consistency. See Supplementary Information for full experimental details. d.r., diastereomeric ratio. #d.r. was determined by 1H NMR.
Extended Data Fig. 2 Decarboxylative sp3 C–N couplings with a series of nitrogen heterocycles.
Various nitrogen heterocycles, including indazoles, azaindoles, indoles and pyrazoles, can cross-couple with carboxylic acids with good efficiency. All yields are isolated. All reactions were replicated at least twice for consistency. See Supplementary Information for full experimental details. *Single regioisomer.
Extended Data Fig. 3 Decarboxylative sp3 C–N couplings with a series of nitrogen nucleophiles.
Various nitrogen nucleophiles, including nitrogen heterocycles, anilines, sulfonamides and amides, can cross-couple with carboxylic acids with good efficiency. All yields are isolated unless otherwise noted. All reactions were replicated at least twice for consistency. See Supplementary Information for full experimental details. #Yield was determined by 19F NMR with an internal standard. &Yield was determined by gas-chromatography analysis with an internal standard. *Single regioisomer.
Extended Data Fig. 4 Decarboxylative sp3 C–N couplings with a series of pharmaceutical compounds.
Several drug molecules can cross-couple with carboxylic acids with good efficiency. All yields are isolated. All reactions were replicated at least twice for consistency. See Supplementary Information for full experimental details. *Single regioisomer.
Supplementary information
Supplementary Information
This file contains supplementary information 1-10
Rights and permissions
About this article
Cite this article
Liang, Y., Zhang, X. & MacMillan, D.W.C. Decarboxylative sp3 C–N coupling via dual copper and photoredox catalysis. Nature 559, 83–88 (2018). https://doi.org/10.1038/s41586-018-0234-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-018-0234-8
This article is cited by
-
Tunable molecular editing of indoles with fluoroalkyl carbenes
Nature Chemistry (2024)
-
N-glycoside synthesis through combined copper- and photoredox-catalysed N-glycosylation of N-nucleophiles
Nature Synthesis (2024)
-
Metallaphotoredox-enabled aminocarboxylation of alkenes with CO2
Nature Catalysis (2023)
-
General access to cubanes as benzene bioisosteres
Nature (2023)
-
Bismuth radical catalysis in the activation and coupling of redox-active electrophiles
Nature Chemistry (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.