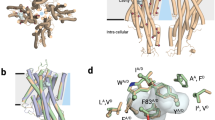
Abstract
The olfactory system must recognize and discriminate amongst an enormous variety of chemicals in the environment. To contend with such diversity, insects have evolved a family of odorant-gated ion channels comprised of a highly conserved co-receptor (Orco) and a divergent odorant receptor (OR) that confers chemical specificity. Here, we present the single-particle cryo-electron microscopy structure of an Orco homomer from the parasitic fig wasp Apocrypta bakeri at 3.5 Å resolution, providing structural insight into this receptor family. Orco possesses a novel channel architecture, with four subunits symmetrically arranged around a central pore that diverges into four lateral conduits that open to the cytosol. The Orco tetramer has few inter-subunit interactions within the membrane and is bound together by a small cytoplasmic anchor domain. The minimal sequence conservation among ORs maps largely to the pore and anchor domain, shedding light on how the architecture of this receptor family accommodates its remarkable sequence diversity and facilitates the evolution of odour tuning.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Mora, C., Tittensor, D. P., Adl, S., Simpson, A. G. B. & Worm, B. How many species are there on Earth and in the ocean? PLoS Biol. 9, e1001127 (2011).
Hansson, B. S. & Stensmyr, M. C. Evolution of insect olfaction. Neuron 72, 698–711 (2011).
Bargmann, C. I. Comparative chemosensation from receptors to ecology. Nature 444, 295–301 (2006).
Kaupp, U. B. Olfactory signalling in vertebrates and insects: differences and commonalities. Nat. Rev. Neurosci. 11, 188–200 (2010).
Malnic, B., Hirono, J., Sato, T. & Buck, L. B. Combinatorial receptor codes for odors. Cell 96, 713–723 (1999).
Wang, J. W., Wong, A. M., Flores, J., Vosshall, L. B. & Axel, R. Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell 112, 271–282 (2003).
Sato, K. et al. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature 452, 1002–1006 (2008).
Wicher, D. et al. Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature 452, 1007–1011 (2008).
Missbach, C. et al. Evolution of insect olfactory receptors. eLife 3, e02115 (2014).
Ioannidis, P. et al. Genomic features of the damselfly Calopteryx splendens representing a sister clade to most insect orders. Genome Biol. Evol. 9, 415–430 (2017).
McKenzie, S. K., Fetter-Pruneda, I., Ruta, V. & Kronauer, D. J. C. Transcriptomics and neuroanatomy of the clonal raider ant implicate an expanded clade of odorant receptors in chemical communication. Proc. Natl Acad. Sci. USA 113, 14091–14096 (2016).
Vosshall, L. B. Olfaction in Drosophila. Curr. Opin. Neurobiol. 10, 498–503 (2000).
Vosshall, L. B., Wong, A. M. & Axel, R. An olfactory sensory map in the fly brain. Cell 102, 147–159 (2000).
Larsson, M. C. et al. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron 43, 703–714 (2004).
Yan, H. et al. An engineered orco mutation produces aberrant social behavior and defective neural development in ants. Cell 170, 736–747.e9 (2017).
Trible, W. et al. orco mutagenesis causes loss of antennal lobe glomeruli and impaired social behavior in ants. Cell 170, 727–735.e10 (2017).
DeGennaro, M. et al. orco mutant mosquitoes lose strong preference for humans and are not repelled by volatile DEET. Nature 498, 487–491 (2013).
Jones, P. L., Pask, G. M., Rinker, D. C. & Zwiebel, L. J. Functional agonism of insect odorant receptor ion channels. Proc. Natl Acad. Sci. USA 108, 8821–8825 (2011).
Lu, B. et al. Expression and evolutionary divergence of the non-conventional olfactory receptor in four species of fig wasp associated with one species of fig. BMC Evol. Biol. 9, 43 (2009).
Benton, R., Sachse, S., Michnick, S. W. & Vosshall, L. B. Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol. 4, e20 (2006).
Jones, W. D., Nguyen, T.-A. T., Kloss, B., Lee, K. J. & Vosshall, L. B. Functional conservation of an insect odorant receptor gene across 250 million years of evolution. Curr. Biol. 15, R119–R121 (2005).
Hopf, T. A. et al. Amino acid coevolution reveals three-dimensional structure and functional domains of insect odorant receptors. Nat. Commun. 6, 6077 (2015).
Corcoran, J. A., Sonntag, Y., Andersson, M. N., Johanson, U. & Löfstedt, C. Endogenous insensitivity to the Orco agonist VUAA1 reveals novel olfactory receptor complex properties in the specialist fly Mayetiola destructor. Sci. Rep. 8, 3489 (2018).
Yang, K., Huang, L.-Q., Ning, C. & Wang, C.-Z. Two single-point mutations shift the ligand selectivity of a pheromone receptor between two closely related moth species. eLife 6, 155 (2017).
Hughes, D. T., Wang, G., Zwiebel, L. J. & Luetje, C. W. A determinant of odorant specificity is located at the extracellular loop 2-transmembrane domain 4 interface of an Anopheles gambiae odorant receptor subunit. Chem. Senses 39, 761–769 (2014).
Nichols, A. S. & Luetje, C. W. Transmembrane segment 3 of Drosophila melanogaster odorant receptor subunit 85b contributes to ligand-receptor interactions. J. Biol. Chem. 285, 11854–11862 (2010).
Leary, G. P. et al. Single mutation to a sex pheromone receptor provides adaptive specificity between closely related moth species. Proc. Natl Acad. Sci. USA 109, 14081–14086 (2012).
Long, S. B., Tao, X., Campbell, E. B. & MacKinnon, R. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature 450, 376–382 (2007).
Liao, M., Cao, E., Julius, D. & Cheng, Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature 504, 107–112 (2013).
Baconguis, I., Bohlen, C. J., Goehring, A., Julius, D. & Gouaux, E. X-ray structure of acid-sensing ion channel 1-snake toxin complex reveals open state of a Na+-selective channel. Cell 156, 717–729 (2014).
Gonzales, E. B., Kawate, T. & Gouaux, E. Pore architecture and ion sites in acid-sensing ion channels and P2X receptors. Nature 460, 599–604 (2009).
Pask, G. M., Jones, P. L., Rützler, M., Rinker, D. C. & Zwiebel, L. J. Heteromeric Anopheline odorant receptors exhibit distinct channel properties. PLoS One 6, e28774 (2011).
Nakagawa, T., Pellegrino, M., Sato, K., Vosshall, L. B. & Touhara, K. Amino acid residues contributing to function of the heteromeric insect olfactory receptor complex. PLoS One 7, e32372 (2012).
Landau, M. et al. ConSurf 2005: the projection of evolutionary conservation scores of residues on protein structures. Nucleic Acids Res. 33, W299–W302 (2005).
Hallem, E. A. & Carlson, J. R. Coding of odors by a receptor repertoire. Cell 125, 143–160 (2006).
Goehring, A. et al. Screening and large-scale expression of membrane proteins in mammalian cells for structural studies. Nat. Protoc. 9, 2574–2585 (2014).
Pédelacq, J.-D., Cabantous, S., Tran, T., Terwilliger, T. C. & Waldo, G. S. Engineering and characterization of a superfolder green fluorescent protein. Nat. Biotechnol. 24, 79–88 (2006).
Gasteiger, E. et al. in The Proteomics Protocols Handbook 571–607 (Humana, New York, 2005).
Mastronarde, D. N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36–51 (2005).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Rohou, A. & Grigorieff, N. CTFFIND4: Fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Zhang, K. Gautomatch https://www.mrc-lmb.cam.ac.uk/kzhang/Gautomatch/ (2016).
Fernandez-Leiro, R. & Scheres, S. H. W. A pipeline approach to single-particle processing in RELION. Acta Crystallogr. D Struct. Biol. 73, 496–502 (2017).
Tang, G. et al. EMAN2: an extensible image processing suite for electron microscopy. J. Struct. Biol. 157, 38–46 (2007).
Grigorieff, N. Frealign: an exploratory tool for single-particle cryo-EM. Methods Enzymol. 579, 191–226 (2016).
Rosenthal, P. B. & Henderson, R. Optimal determination of particle orientation, absolute hand, and contrast loss in single-particle electron cryomicroscopy. J. Mol. Biol. 333, 721–745 (2003).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Holm, L. & Laakso, L. M. Dali server update. Nucleic Acids Res. 44, W351–W355 (2016).
The PyMOL Molecular Graphics System, Version 2.0 Schrödinger, LLC.
Smart, O. S., Neduvelil, J. G., Wang, X., Wallace, B. A. & Sansom, M. S. HOLE: a program for the analysis of the pore dimensions of ion channel structural models. J. Mol. Graph. 14, 354–360, 376 (1996).
Chen, T.-W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
Sievers, F. et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 7, 539 (2011).
Goujon, M. et al. A new bioinformatics analysis tools framework at EMBL-EBI. Nucleic Acids Res. 38, W695–W699 (2010).
Hill, C. A. et al. G protein-coupled receptors in Anopheles gambiae. Science 298, 176–178 (2002).
Vosshall, L. B., Amrein, H., Morozov, P. S., Rzhetsky, A. & Axel, R. A spatial map of olfactory receptor expression in the Drosophila antenna. Cell 96, 725–736 (1999).
Robertson, H. M., Gadau, J. & Wanner, K. W. The insect chemoreceptor superfamily of the parasitoid jewel wasp Nasonia vitripennis. Insect Mol. Biol. 19 (Suppl 1), 121–136 (2010).
Pelletier, J., Xu, P., Yoon, K. S., Clark, J. M. & Leal, W. S. Odorant receptor-based discovery of natural repellents of human lice. Insect Biochem. Mol. Biol. 66, 103–109 (2015).
Katoh, K., Misawa, K., Kuma, K. & Miyata, T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 30, 3059–3066 (2002).
Katoh, K., Rozewicki, J. & Yamada, K. D. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 30, 3059 (2017).
Ashkenazy, H. et al. ConSurf 2016: an improved methodology to estimate and visualize evolutionary conservation in macromolecules. Nucleic Acids Res. 44, W344–W350 (2016).
Wittig, I., Braun, H.-P. & Schägger, H. Blue native PAGE. Nat. Protoc. 1, 418–428 (2006).
Hou, X., Pedi, L., Diver, M. M. & Long, S. B. Crystal structure of the calcium release-activated calcium channel Orai. Science 338, 1308–1313 (2012).
Whicher, J. R. & MacKinnon, R. Structure of the voltage-gated K+ channel Eag1 reveals an alternative voltage sensing mechanism. Science 353, 664–669 (2016).
Acknowledgements
We thank R. Axel, R. MacKinnon, B. Noro and L. Vosshall for advice on the manuscript, A. Siliciano and C. Monnier for technical advice and preliminary biochemistry, other members of the Ruta laboratory for discussions, and R. Boggavarapu for help with initial negative-stain EM experiments. We are grateful to M. Ebrahim and J. Sotiris at The Rockefeller University Evelyn Gruss Lipper Cryo-Electron Microscopy Resource Center for assistance with microscope operation, F. Weis-Garcia and S. L. Bourne at the MSKCC Antibody & Bioresource Core Facility for hybridoma generation, and R. Lavoisier and C. Adura at The Rockefeller University High Throughput & Spectroscopy Resource Center for support on functional assays. This work was supported by a Helmsley Postdoctoral Fellowship (to K.H.K.) and a Sinsheimer Foundation Award, the National Institutes of Health (R01-AI103171-01A1) and New York Stem Cell Foundation Robertson Neuroscience Investigator Award (to V.R.).
Reviewer information
Nature thanks R. Benton, E. Gouaux and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
J.A.B. and M.A.K. expressed and purified Orco, screened monoclonal antibody lines and purified Fab fragments. J.A.B. and K.H.K. collected and analysed cryo-EM data with input from T.W. J.A.B. built and refined the Orco model and performed ITC measurements. J.d.M. performed electrophysiology experiments. J.A.R. performed calcium imaging assays. V.R. contributed to all aspects of the project and wrote the paper with J.A.B. and input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Ligand-gated signalling of A. bakeri and A. gambiae Orcos.
a, Fluorescence changes in HEK293 cells transfected with a genetically encoded Ca2+ indicator, GCaMP6s, and Orco from A. bakeri (Abak; closed circles) or A. gambiae (Agam; open circles) alone, or with an A. gambiae OR. Dose–response curves were obtained by titrating with VUAA1 or the cognate odour of the OR. b, Average fitted Hill equation parameters from N number of independent replicates. EC50 is the concentration for half-maximal response and n is the Hill coefficient (both mean ± s.d.). 245-T is 2,4,5-trimethylthiazole.
Extended Data Fig. 2 Electrophysiological characterization of Orco.
a, Example VUAA1 dose–response curve using whole-cell current from a HEK293 cell expressing Orco. I/Imax is the measured current relative to the maximum current obtained at the highest concentration of VUAA1. The titration experiment was repeated using four independent cells with equivalent results. The average fitted Hill equation parameters are (mean ± s.d.): EC50 = 64 ± 7 μM, n = 3.5 ± 0.5. b, Inward whole-cell current for a subset of the VUAA1 concentrations used in a (held at –80 mV). c, VUAA1-evoked Orco currents recorded from an outside-out membrane patch from a HEK293 cell expressing Orco (held at –140 mV). Inset (0.9 s) highlights single channel openings. d, Amplitude histogram determined from the inset in c. Fitted Gaussian distributions for single-channel Orco current (red) and baseline current (black) are shown. e, Amplitude histograms were obtained at multiple voltages and the single-channel conductance of 10.6 ± 0.6 pS was determined from the slope of the current-voltage plot (mean ± s.d. for 2–6 data points per voltage from 9 patches). The specific numbers of replicates at each voltage were: 5 (–140 mV), 4 (–130 mV), 5 (–120 mV), 3 (–110 mV), 6 (–100 mV), 4 (–90 mV), 4 (–80 mV), 3 (–70 mV), 2 (–60 mV).
Extended Data Fig. 3 Stoichiometry and ligand binding of the Orco homotetramer.
a, Western blot of cross-linked Orco in transfected HEK293 cells. SDS–PAGE showing a ladder of four bands that appears with both GFP-tagged and untagged Orco after treatment with increasing concentration of the amine cross-linker disuccinimidyl suberate (DSS). Monomer (m) and tetramer (t) bands are indicated. DSS concentrations were (μM): 0, 25, 75, 125, 250, 2,500. b, Western blot of a Blue Native (BN)-PAGE63 gel of the same samples as in a showing that tetrameric Orco is present in all samples and higher-order aggregates are not induced by cross-linking. c, d, Western blot of Orco extracted with increasing concentrations of detergent (LMNG) showed gradual loss of the tetrameric species. Addition of CHS (c), porcine brain polar lipid extract (d) (BPL; Anatrace) or the combination of the two stabilized the Orco tetramer. LMNG concentrations were (% w/v): 0.01, 0.05, 0.1, 0.25, 0.5, 1. Concentration of CHS, BPL and the sum of CHS + BPL were added at one-fifth that of LMNG. GFP-labelled Orai (55 kDa)64 was used as a molecular weight marker as it is a hexamer with a similar total size as the Orco–GFP tetramer (340 kDa). The larger apparent molecular weights observed in BN-PAGE gels (b–d) reflect the additional mass of the micelle. Primary antibodies used were: anti-Orco clone 20F7 and anti-GFP (Life Technologies). Each experiment in this figure was repeated three times with similar results. The molecular weight markers on the native gels are approximate: they are from a separate gel run under the same conditions (see Supplementary Data). e, f, Representative baseline-corrected isothermal titration calorimetry (ITC) data for Orco (e, 11 μM) and Orco–Fab complex (f, 10 μM) titrated with VUAA1. Integrated heats and fitted single-site binding isotherms are shown at the bottom. The number of binding sites per monomer was fixed at 1 and the dissociation constant (Kd), enthalpy of binding (ΔH), and heat associated with sample dilution (ΔQdil) were fit. The experiments were repeated three times each using Orco samples obtained from independent purifications. The average fitted thermodynamic parameters are as follows (mean ± s.d.). Orco: Kd = 13 ± 1 μM, ΔH = –8.3 ± 0.6 kcal/mol, ΔQdil = –1.0 ± 0.1 kcal/mol. Orco–Fab: Kd = 18 ± 2 μM, ΔH = –9.7 ± 0.4 kcal/mol, ΔQdil = –1.0 ± 0.2 kcal/mol.
Extended Data Fig. 4 Cryo-EM data analysis.
a, A representative motion-corrected micrograph showing the distribution of Orco–Fab single particles (three particles are circled). Scale bar, 50 nm. b, 2D averages of classes selected for further processing. c, Initial density map from 3D refinement in RELION using all of the particles in b with C4 symmetry imposed. d, Soft mask and final density map after refinement in Frealign. e, Fourier shell correlation (FSC) curves for the final cryo-EM density maps. The horizontal dashed line represents the 0.143 cutoff value. f, Orientation distribution of the particles included in the final 3D map of the Orco–Fab complex in d (as reported by RELION). g, Approximate local resolution of the entire Orco–Fab density map (left) and only Orco (right). h, Cryo-EM densities for the modelled regions are shown as grey mesh. Orco models are drawn as sticks with carbon atoms coloured according to Fig. 2, and oxygen, nitrogen and sulfur atoms coloured red, blue and yellow, respectively.
Extended Data Fig. 5 Potential extracellular facing odour-binding pocket.
a, Side view of Orco highlighting the location of residues in Orco or ORs that, when mutated, alter ligand-binding specificity. Orco residues that are equivalent to point mutations that alter odour specificity in Helicoverpa assaulta OR14b24, A. gambiae OR1525, D. melanogaster OR85b26 or Ostrinia furnacalis OR327 are shown as red spheres. Residues required for VUAA1 sensitivity in Orco23 are shown as blue spheres. In Orco, the S3–S4 extracellular loop is positioned above the pocket. b, A 15 Å cross-section through the pocket from a.
Extended Data Fig. 6 Inter-subunit interactions in Orco and Kv channels.
a, Top views of Orco (left) and Kv10.1 (Eag1)65 (right) highlighting the overall organization of these tetrameric cation channels. In this Kv channel, the pore and voltage-domains are not domain-swapped and so it more closely resembles the quaternary structure of Orco compared to other Kv channels. b, c, Top (b) and side (c) views showing inter-subunit interactions. Residues within 5 Å of a different subunit within the transmembrane region are shown as coloured spheres (16 residues in Orco, 58 residues in Kv10.1). In Orco, residues at subunit interfaces in the anchor domain are grey spheres (35 residues). The extracellular and intracellular domains of Kv10.1 are not shown.
Extended Data Fig. 7 Reversal potentials and ion-permeability ratios.
a, b, VUAA1-evoked whole-cell current from HEK293 cells expressing Orco with 150 mM intracellular CsCl and 150 mM extracellular NaCl (a, blue), or 100 mM extracellular CaCl2 (b, red). c, Summary of reversal potentials (Erev) and permeability ratios (Pion/PCs) for wild-type and mutant Orco and Orco–OR complexes measured under bi-ionic conditions (mean ± s.d.). Erev were measured using N independent cells and corrected for the measured junction potential. Pion/PCs were calculated from Erev using Goldman–Hodgkin–Katz equations (see Methods) with their errors determined by propagation of the standard deviations. Three one-way ANOVA tests were performed using these Erev data followed by Tukey–Kramer multiple comparison tests. (1) Orco selectivity of Na+, K+, Ca2+ and Mg2+ (P values are from comparisons to Na+ Erev). (2) Wild-type and mutant Orco Na+ and Ca2+ selectivity (P values are from comparisons to wild-type Orco with the same ion, Na+ or Ca2+, as appropriate). (3) Orco homomer and Orco–OR heteromer Na+ and Ca2+ selectivity (P values are from comparisons to the Orco homomer with the same ion, Na+ or Ca2+, as appropriate). 245-T is 2,4,5-trimethylthiazole.
Extended Data Fig. 8 Cell-based GCaMP assay.
a, b, Relative fluorescence changes in HEK293 cells transfected with GCaMP plus wild-type (WT) or mutant Orco alone (a, titrated with VUAA1) or in the presence of A. gambiae OR65 (b, titrated with eugenol). Plots using the same symbols were collected on the same day. c, d, Fitted Hill equation parameters for Orco and Orco–OR65 (mean ± s.d.). The assays were repeated N independent times to obtain the maximum ΔF/Fo response, concentration for half-maximal response (EC50) and Hill coefficient (n). (ΔF/Fo)norm is the fitted maximum ΔF/Fo response relative to a wild-type control Orco or Orco–OR65 experiment from the same plate (for mutations that could not be accurately fit, the measured maximum ΔF/Fo response was used). Two one-way ANOVA tests were performed using these EC50 data followed by Tukey–Kramer multiple comparison tests. (1) Wild-type and mutant Orco homomers (P values are from comparisons to wild-type Orco). (2) Wild-type and mutant Orco co-transfected with OR65 (P values are from comparisons to wild-type Orco with OR65). *Fitted parameters for Orco T465A or S458A homomers + VUAA1 could not be obtained as the dose–response curves did not saturate: a lower-bound estimate for their EC50 is approximately 3 × 10−4 M. **No binding was observed for Orco Y466A in the presence of OR65.
Extended Data Fig. 9 Conservation of Orco and OR sequences.
a, Orco (left) and OR (right) conservation scores mapped onto the structure of Orco. b, Consensus sequences from Orco and OR amino-acid alignments (see Supplementary Data) are aligned to A. bakeri Orco. In total, 176 Orco sequences and 361 OR sequences were used in the alignments. The per cent identities (bar height) and ConSurf62 conservation scores (bar colour) are plotted for each consensus sequence. Only residues that align to A. bakeri Orco are included.
Supplementary information
Supplementary Figure
This file contains full gel images from Extended Data Fig. 3.
Supplementary Data 1
Sequence alignment of Orco proteins.
Supplementary Data 2
Sequence alignment of OR proteins.
Rights and permissions
About this article
Cite this article
Butterwick, J.A., del Mármol, J., Kim, K.H. et al. Cryo-EM structure of the insect olfactory receptor Orco. Nature 560, 447–452 (2018). https://doi.org/10.1038/s41586-018-0420-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-018-0420-8
This article is cited by
-
The molecular basis of sugar detection by an insect taste receptor
Nature (2024)
-
Identification of an adult attractant for Anomala corpulenta by the reverse chemical ecology approach
Journal of Pest Science (2024)
-
Insights of the Rhynchophorus ferrugineus chemosensory system towards controlling its palm infestation problem: Progress from Omics research and technologies
International Journal of Tropical Insect Science (2024)
-
Structural basis of amine odorant perception by a mammal olfactory receptor
Nature (2023)
-
Structural basis of odorant recognition by a human odorant receptor
Nature (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.