Abstract
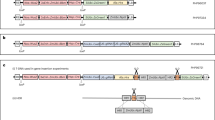
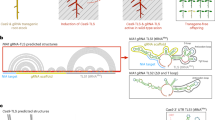
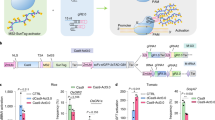
The potential of genome editing to improve the agronomic performance of crops is often limited by low plant regeneration efficiencies and few transformable genotypes. Here, we show that expression of a fusion protein combining wheat GROWTH-REGULATING FACTOR 4 (GRF4) and its cofactor GRF-INTERACTING FACTOR 1 (GIF1) substantially increases the efficiency and speed of regeneration in wheat, triticale and rice and increases the number of transformable wheat genotypes. GRF4–GIF1 transgenic plants were fertile and without obvious developmental defects. Moreover, GRF4–GIF1 induced efficient wheat regeneration in the absence of exogenous cytokinins, which facilitates selection of transgenic plants without selectable markers. We also combined GRF4–GIF1 with CRISPR–Cas9 genome editing and generated 30 edited wheat plants with disruptions in the gene Q (AP2L-A5). Finally, we show that a dicot GRF–GIF chimera improves regeneration efficiency in citrus, suggesting that this strategy can be applied to dicot crops.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Accession numbers and gene names are available in the phylogenetic tree in Supplementary Fig. 1. All wheat gene names are based on genome release RefSeq v1.0. The raw data for the different experiments are available in Supplementary Tables 3, 4, 6 and 7. The steps for the generation of the different vectors and the transformation protocols are described in the Methods. The following vectors will be available through Addgene (http://www.addgene.org/): JD553-wheat GRF4–GIF1 in pDONR, JD633-wheat GRF4–GIF1 in the CRISPR vector, JD630-Vitis GRF4–GIF1 in pDONR, JD638-Vitis miR396-resistant GRF4–GIF1 in pDONR, JD689-Citrus GRF4–GIF1 in pDONR, JD690-Citrus GRF4–GIF1 in pGWB14, JD631-Vitis GRF4–GIF1 in pGWB14 and JD639-Vitis miR396-resistant GRF4–GIF1 in pGWB14.
References
Lotan, T. et al. Arabidopsis LEAFY COTYLEDON1 is sufficient to induce embryo development in vegetative cells. Cell 93, 1195–1205 (1998).
Lowe, K. et al. In Plant Biotechnology 2002 and Beyond: Proceedings of the 10th International Association for Plant Tissue Culture and Biotechnology Congress (ed. Vasil, I. K.) 283–284 (Springer, 2003).
Stone, S. L. et al. LEAFY COTYLEDON2 encodes a B3 domain transcription factor that induces embryo development. Proc. Natl Acad. Sci. USA 98, 11806–11811 (2001).
Zuo, J. R., Niu, Q. W., Frugis, G. & Chua, N. H. The WUSCHEL gene promotes vegetative-to-embryonic transition in Arabidopsis. Plant J. 30, 349–359 (2002).
Boutilier, K. et al. Ectopic expression of BABY BOOM triggers a conversion from vegetative to embryonic growth. Plant Cell 14, 1737–1749 (2002).
Gordon-Kamm, B. et al. Using morphogenic genes to improve recovery and regeneration of transgenic plants. Plants 8, 38 (2019).
Lowe, K. et al. Rapid genotype “independent” Zea mays L. (maize) transformation via direct somatic embryogenesis. In Vitro Cell. Dev. Biol. Plant 54, 240–252 (2018).
Lowe, K. et al. Morphogenic regulators Baby boom and Wuschel improve monocot transformation. Plant Cell 28, 1998–2015 (2016).
Maher, M. F. et al. Plant gene editing through de novo induction of meristems. Nat. Biotechnol. 38, 84–89 (2020).
Omidbakhshfard, M. A., Proost, S., Fujikura, U. & Mueller-Roeber, B. Growth-regulating factors (GRFs): a small transcription factor family with important functions in plant biology. Mol. Plant 8, 998–1010 (2015).
Kim, J. H., Choi, D. S. & Kende, H. The AtGRF family of putative transcription factors is involved in leaf and cotyledon growth in Arabidopsis. Plant J. 36, 94–104 (2003).
Kim, J. H. & Kende, H. A transcriptional coactivator, AtGIF1, is involved in regulating leaf growth and morphology in Arabidopsis. Proc. Natl Acad. Sci. USA 101, 13374–13379 (2004).
Horiguchi, G., Kim, G. T. & Tsukaya, H. The transcription factor AtGRF5 and the transcription coactivator AN3 regulate cell proliferation in leaf primordia of Arabidopsis thaliana. Plant J. 43, 68–78 (2005).
Debernardi, J. M., Rodriguez, R. E., Mecchia, M. A. & Palatnik, J. F. Functional specialization of the plant miR396 regulatory network through distinct microRNA–target interactions. PLoS Genet. 8, e1002419 (2012).
Debernardi, J. M. et al. Post-transcriptional control of GRF transcription factors by microRNA miR396 and GIF co-activator affects leaf size and longevity. Plant J. 79, 413–426 (2014).
Vercruyssen, L. et al. ANGUSTIFOLIA3 binds to SWI/SNF chromatin remodeling complexes to regulate transcription during Arabidopsis leaf development. Plant Cell 26, 210–229 (2014).
Liebsch, D. & Palatnik, J. F. MicroRNA miR396, GRF transcription factors and GIF co-regulators: a conserved plant growth regulatory module with potential for breeding and biotechnology. Curr. Opin. Plant Biol. 53, 31–42 (2020).
Li, S. C. et al. The OsmiR396c–OsGRF4–OsGIF1 regulatory module determines grain size and yield in rice. Plant Biotechnol. J. 14, 2134–2146 (2016).
Rodriguez, R. E. et al. Control of cell proliferation in Arabidopsis thaliana by microRNA miR396. Development 137, 103–112 (2010).
He, Z. S. et al. OsGIF1 positively regulates the sizes of stems, leaves, and grains in rice. Front. Plant Sci. 8, 1730 (2017).
Shimano, S. et al. Conserved functional control, but distinct regulation, of cell proliferation in rice and Arabidopsis leaves revealed by comparative analysis of GRF-INTERACTING FACTOR 1 orthologs. Development 145, dev159624 (2018).
Zhang, D. et al. GRF-Interacting Factor1 regulates shoot architecture and meristem determinacy in maize. Plant Cell 30, 360–374 (2018).
Duan, P. et al. Regulation of OsGRF4 by OsmiR396 controls grain size and yield in rice. Nat. Plants 2, 15203 (2015).
Hu, J. et al. A rare allele of GS2 enhances grain size and grain yield in rice. Mol. Plant 8, 1455–1465 (2015).
Che, R. H. et al. Control of grain size and rice yield by GL2-mediated brassinosteroid responses. Nat. Plants 2, 15195 (2016).
Sun, P. Y. et al. OsGRF4 controls grain shape, panicle length and seed shattering in rice. J. Integ. Plant Biol. 58, 836–847 (2016).
Li, S. et al. Modulating plant growth—metabolism coordination for sustainable agriculture. Nature 560, 595–600 (2018).
Rodriguez, R. E. et al. MicroRNA miR396 regulates the switch between stem cells and transit-amplifying cells in Arabidopsis roots. Plant Cell 27, 3354–3366 (2015).
Ishida, Y., Hiei, Y. & Komari, T. In Proceedings of the 12th International Wheat Genetics Symposium (eds. Ogihara, Y. et al.) 167–173 (Springer, 2015).
Richardson, T., Thistleton, J., Higgins, T. J., Howitt, C. & Ayliffe, M. Efficient Agrobacterium transformation of elite wheat germplasm without selection. Plant Cell Tiss. Organ Cult. 119, 647–659 (2014).
Wang, K., Liu, H. Y., Du, L. P. & Ye, X. G. Generation of marker-free transgenic hexaploid wheat via an Agrobacterium-mediated co-transformation strategy in commercial Chinese wheat varieties. Plant Biotechnol. J. 15, 614–623 (2017).
Hayta, S. et al. An efficient and reproducible Agrobacterium-mediated transformation method for hexaploid wheat (Triticum aestivum L.). Plant Methods 15, 121 (2019).
Debernardi, J. M., Lin, H., Chuck, G., Faris, J. D. & Dubcovsky, J. microRNA172 plays a crucial role in wheat spike morphogenesis and grain threshability. Development 144, 1966–1975 (2017).
Kong, J. et al. Overexpression of the transcription factor GROWTH-REGULATING FACTOR5 improves transformation of dicot and monocot species. Front. Plant Sci. https://doi.org/10.3389/fpls.2020.572319 (2020).
Wang, W., Akhunova, A., Chao, S. & Akhunov, E. Optimizing multiplex CRISPR/Cas9-based genome editing for wheat. Preprint at bioRxiv https://doi.org/10.1101/051342 (2016).
Chern, M. S. et al. Evidence for a disease-resistance pathway in rice similar to the NPR1-mediated signaling pathway in Arabidopsis. Plant J. 27, 101–113 (2001).
Acknowledgements
This project was supported by the Howard Hughes Medical Institute, NRI Competitive Grant 2017-67007-25939 from the USDA National Institute of Food and Agriculture (NIFA) and the International Wheat Partnership Initiative (IWYP). J.F.P. acknowledges support from the Argentinean Research Council (CONICET); Agencia Nacional de Promoción de la Investigación, el Desarrollo Tecnológico y la Innovación; and International Centre for Genetic Engineering and Biotechnology, grant ICGEB/ARG17-01. P.R. was supported by NIH grant GM122968. S.H. acknowledges support from the Biotechnology and Biological Sciences Research Council Genes in the Environment Institute Strategic Programme BB/P013511/1. J.M.D. was supported by a fellowship (LT000590/2014-L) from the Human Frontier Science Program. M.F.E. is a Latin American Fellow in the Biomedical Sciences, supported by the Pew Charitable Trusts. We thank Y. Wang (Chinese Academy of Sciences, Beijing) for the pYP25F binary vector, M. Padilla, R. Rasia, G. Rabasa, B. Van Bockern and M. Smedley for excellent technical support and C. Uauy for coordinating the testing of the GRF4–GIF1 chimera at the John Innes Centre.
Author information
Authors and Affiliations
Contributions
J.M.D. contributed to the investigation, methodology, formal analysis, writing and editing. D.M.T. contributed to the investigation, supervision, methodology, project administration, funding acquisition, writing and editing. J.F.P. contributed to study conceptualization, writing and editing. M.F.E. contributed to the experiments involving rice, writing and editing. S.H. performed wheat transformation experiments at the John Innes Centre. P.R. supervised the experiments involving rice and contributed to writing and editing. J.D. contributed to study conceptualization, formal analysis, supervision, project administration, funding acquisition, writing and editing.
Corresponding author
Ethics declarations
Competing interests
J.F.P. and J.M.D. are co-inventors in patent US2017/0362601A1 that describes the use of chimeric GRF–GIF proteins with enhanced effects on plant growth (Universidad Nacional de Rosario Consejo Nacional de Investigaciones Científicas y Técnicas). J.F.P., J.D., D.M.T. and J.M.D. are co-inventors in UC Davis provisional patent application 62/873,123 that describes the use of GRF–GIF chimeras to enhance regeneration efficiency in plants. Vectors are freely available for research, but commercial applications may require a paid nonexclusive license. There is a patent application from KWS/BASF (WO 2019/134884 A1) for improved plant regeneration using Arabidopsis GRF5 and grass GRF1 homologs. None of the authors of this manuscript is part of the KWS/BASF patent or is related to these companies. The KWS/BASF patent focuses on a different cluster of GRF genes than the one described in our study and does not incorporate the GIF1 cofactor or the generation of GRF–GIF chimeras.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–6, Tables 1–8 and Material Transfer Agreement
Rights and permissions
About this article
Cite this article
Debernardi, J.M., Tricoli, D.M., Ercoli, M.F. et al. A GRF–GIF chimeric protein improves the regeneration efficiency of transgenic plants. Nat Biotechnol 38, 1274–1279 (2020). https://doi.org/10.1038/s41587-020-0703-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41587-020-0703-0
This article is cited by
-
Genome-wide molecular evolution analysis of the GRF and GIF gene families in Plantae (Archaeplastida)
BMC Genomics (2024)
-
Improving Sedum plumbizincicola genetic transformation with the SpGRF4–SpGIF1 gene and the self-excision CRE/LoxP system
Planta (2024)
-
Genome editing based trait improvement in crops: current perspective, challenges and opportunities
The Nucleus (2024)
-
Targeted genome editing for cotton improvement: prospects and challenges
The Nucleus (2024)