Abstract
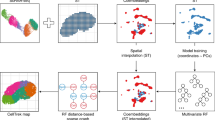
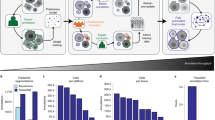
Despite advances in three-dimensional (3D) imaging, it remains challenging to profile all the cells within a large 3D tissue, including the morphology and organization of the many cell types present. Here, we introduce eight-color, multispectral, large-scale single-cell resolution 3D (mLSR-3D) imaging and image analysis software for the parallelized, deep learning–based segmentation of large numbers of single cells in tissues, called segmentation analysis by parallelization of 3D datasets (STAPL-3D). Applying the method to pediatric Wilms tumor, we extract molecular, spatial and morphological features of millions of cells and reconstruct the tumor’s spatio-phenotypic patterning. In situ population profiling and pseudotime ordering reveals a highly disorganized spatial pattern in Wilms tumor compared to healthy fetal kidney, yet cellular profiles closely resembling human fetal kidney cells could be observed. In addition, we identify previously unreported tumor-specific populations, uniquely characterized by their spatial embedding or morphological attributes. Our results demonstrate the use of combining mLSR-3D and STAPL-3D to generate a comprehensive cellular map of human tumors.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Data are publicly available. Processed results (that is, cells × features matrices and clustering and pseudotime results) and imaging data are made available through public repositories for which the links are posted on the STAPL-3D GitHub page.
Code availability
We provide the STAPL-3D framework as a Python package on github (https://github.com/RiosGroup/STAPL3D).
References
Coutu, D. L., Kokkaliaris, K. D., Kunz, L. & Schroeder, T. Multicolor quantitative confocal imaging cytometry. Nat. Methods 15, 39–46 (2018).
Li, W., Germain, R. N. & Gerner, M. Y. High-dimensional cell-level analysis of tissues with Ce3D multiplex volume imaging. Nat. Protoc. 14, 1708–1733 (2019).
Rios, A. C. et al. Intraclonal plasticity in mammary tumors revealed through large-scale single-cell resolution 3D imaging. Cancer Cell 35, 618–632.e6 (2019).
Segovia-Miranda, F. et al. Three-dimensional spatially resolved geometrical and functional models of human liver tissue reveal new aspects of NAFLD progression. Nat. Med. 25, 1885–1893 (2019).
Steinert, E. M. et al. Quantifying memory CD8 T cells reveals regionalization of immunosurveillance. Cell 161, 737–749 (2015).
Mosaliganti, K. R., Noche, R. R., Xiong, F., Swinburne, I. A. & Megason, S. G. ACME: automated cell morphology extractor for comprehensive reconstruction of cell membranes. PLoS Comput. Biol. 8, e1002780 (2012).
Stegmaier, J. et al. Real-time three-dimensional cell segmentation in large-scale microscopy data of developing embryos. Dev. Cell 36, 225–240 (2016).
McQuin, C. et al. CellProfiler 3.0: Next-generation image processing for biology. PLoS Biol. 16, e2005970 (2018).
Dunn, K. W. et al. DeepSynth: Three-dimensional nuclear segmentation of biological images using neural networks trained with synthetic data. Sci. Rep. 9, 18295 (2019).
Wolny, A. et al. Accurate and versatile 3D segmentation of plant tissues at cellular resolution. Elife 9, 1–34 (2020).
Weigert, M., Schmidt, U., Haase, R., Sugawara, K. & Myers, G. Star-convex polyhedra for 3D object detection and segmentation in microscopy. in Proceedings - 2020 IEEE Winter Conference on Applications of Computer Vision, WACV 2020 3655–3662 (2020). https://doi.org/10.1109/WACV45572.2020.9093435
Zhao, S. et al. Cellular and molecular probing of intact human organs. Cell 180, 796–812.e19 (2020).
Kraus, B., Ziegler, M. & Wolff, H. Linear fluorescence unmixing in cell biological research. Mod. Res. Educ. Top. Microsc. 2, 863–872 (2007).
Valm, A. M. et al. Applying systems-level spectral imaging and analysis to reveal the organelle interactome. Nature 546, 162–167 (2017).
Hochane, M. et al. Single-cell transcriptomics reveals gene expression dynamics of human fetal kidney development. PLoS Biol. 17, e3000152 (2019).
Tustison, N. J. et al. N4ITK: Improved N3 bias correction. IEEE Trans. Med. Imaging 29, 1310–1320 (2010).
Berg, S. et al. Ilastik: interactive machine learning for (bio)image analysis. Nat. Methods 16, 1226–1232 (2019).
Gut, G., Herrmann, M. D. & Pelkmans, L. Multiplexed protein maps link subcellular organization to cellular states. Science 80, 361 (2018).
Young, M. D. et al. Single-cell transcriptomes from human kidneys reveal the cellular identity of renal tumors. Science 361, 594–599 (2018).
Young, M. D. et al. Single cell derived mRNA signals across human kidney tumors. Preprint at bioRxiv https://doi.org/10.1101/2020.03.19.998815 (2020).
McInnes, L. PCA, t-SNE, and UMAP: modern approaches to dimension reduction. PyData Conference 2018 (2018).
Reinhard, H. et al. Outcome of relapses of nephroblastoma in patients registered in the SIOP/GPOH trials and studies. Oncol. Rep. 20, 463–467 (2008).
Wegert, J. et al. Mutations in the SIX1/2 pathway and the DROSHA/DGCR8 miRNA microprocessor complex underlie high-risk blastemal type Wilms tumors. Cancer Cell 27, 298–311 (2015).
Glaser, A. K. et al. Multi-immersion open-top light-sheet microscope for high-throughput imaging of cleared tissues. Nat. Commun. 10, 2781 (2019).
Stoltzfus, C. R. et al. CytoMAP: a spatial analysis toolbox reveals features of myeloid cell organization in lymphoid tissues. Cell Rep. 31, 107523 (2020).
Merritt, C. R. et al. Multiplex digital spatial profiling of proteins and RNA in fixed tissue. Nat. Biotechnol. 38, 586–599 (2020).
Dekkers, J. F. et al. High-resolution 3D imaging of fixed and cleared organoids. Nat. Protoc. 14, 1756–1771 (2019).
van Ineveld, R. L., Ariese, H. C. R., Wehrens, E. J., Dekkers, J. F. & Rios, A. C. Single-cell resolution three-dimensional imaging of intact organoids. J. Vis. Exp. 2020, 1–8 (2020).
Yushkevich, P. A. et al. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 31, 1116–1128 (2006).
Sauvola, J. & Pietikäinen, M. Adaptive document image binarization. Pattern Recognit. 33, 225–236 (2000).
Klein, S., Staring, M., Murphy, K., Viergever, M. A. & Pluim, J. P. W. Elastix: A toolbox for intensity-based medical image registration. IEEE Trans. Med. Imaging 29, 196–205 (2010).
Dice, L. R. Measures of the amount of ecologic association between species. Ecology 26, 297–302 (1945).
Wei, T. & Simko, V. corrplot. R Package, v. 0.84 (2017).
Basser, P. J. & Pierpaoli, C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J. Magn. Reson. Ser. B 111, 209–219 (1996).
Wolf, F. A., Angerer, P. & Theis, F. J. SCANPY: Large-scale single-cell gene expression data analysis. Genome Biol. 19, 15 (2018).
Traag, V. A., Waltman, L. & van Eck, N. J. From Louvain to Leiden: guaranteeing well-connected communities. Sci. Rep. 9, (2019).
Pedregosa, F. et al. Scikit-learn: Machine learning in Python. J. Mach. Learn. Res. 12, 2825–2830 (2011).
Haghverdi, L., Büttner, M., Wolf, F. A., Buettner, F. & Theis, F. J. Diffusion pseudotime robustly reconstructs lineage branching. Nat. Methods 13, 845–848 (2016).
Acknowledgements
We are grateful for the technical support from the Princess Máxima Center for Pediatric Oncology and Zeiss for imaging support. We acknowledge the Gynaikon Clinic in Rotterdam for their efforts to provide the human fetal material and the laboratory of Hans Clevers and the Hubrecht Organoid Technology (HUB, www.hub4organoids.nl) for access to the breast cancer organoid biobank. We also acknowledge the Utrecht Bioinformatics Center High Performance Computing Facility for data processing infrastructure. We thank R.R. de Krijger for useful discussions. All the imaging was performed at the Princess Máxima Imaging Center. This work was financially supported by the Princess Máxima Center for Pediatric Oncology and St. Baldrick’s Robert J. Arceci International Innovation award. J.F.D. is supported by a VENI grant from the Netherlands Organisation for Scientific Research (NWO). A.C.R is supported by an ERC-starting grant 2019 project no. 804412.
Author information
Authors and Affiliations
Contributions
R.L.v.I. and M.K. contributed equally. M.A. and S.d.B. contributed equally. R.L.v.I. developed mLSR-3D and performed microscopy. M.K. developed STAPL-3D and performed the computational methods. R.L.v.I. and M.K. analyzed the data. S.d.B. assisted with computational analysis. C.M.M. assisted with microscopy. M.B.R. performed mLSR-3D imaging of breast and neuronal tumor tissue, rendered data and made videos. E.J.v.V. performed mLSR-3D imaging of breast and neuronal tumor tissue. M.A. performed quality control and computational analysis. J.F.D. provided organoid and xenograft material. H.R.J. assisted with sample preparation. F.L.B. provided microscopy support. R.H. provided the NCAM nanobody used in this study. S.M.C.d.S.L provided human fetal material. J.F.D., M.A., E.J.v.V., S.d.B., F.L.B. and J.D. provided critical feedback on the work. R.L.v.I., M.K. and A.C.R. designed the study and wrote the manuscript with support from E.J.W., and A.C.R. supervised this work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Biotechnology thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–16 and Tables 1–5.
Supplementary Video 1
Video abstract.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
van Ineveld, R.L., Kleinnijenhuis, M., Alieva, M. et al. Revealing the spatio-phenotypic patterning of cells in healthy and tumor tissues with mLSR-3D and STAPL-3D. Nat Biotechnol 39, 1239–1245 (2021). https://doi.org/10.1038/s41587-021-00926-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41587-021-00926-3
This article is cited by
-
Bridging live-cell imaging and next-generation cancer treatment
Nature Reviews Cancer (2023)
-
Multispectral confocal 3D imaging of intact healthy and tumor tissue using mLSR-3D
Nature Protocols (2022)
-
Resolving the spatial heterogeneity of cancer in 3D
Nature Reviews Cancer (2022)
-
Tissue clearing to examine tumour complexity in three dimensions
Nature Reviews Cancer (2021)