Abstract
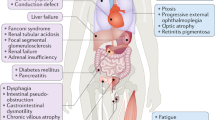
Inherited mitochondrial DNA (mtDNA) diseases were discovered 30 years ago, and their characterization has provided a new perspective on the etiology of the common metabolic and degenerative diseases, cancer, and aging. The maternally inherited mtDNA contains 37 critical bioenergetic genes that are present in hundreds of copies per cell, but the ‘mitochondrial genome’ encompasses an additional 1,000–2,000 nuclear DNA (nDNA) mitochondrial genes. The interaction between these two mitochondrial genetic systems provides explanations for phenomena such as the non-Mendelian transmission of the common ‘complex’ diseases, age-related disease risk and progression, variable penetrance and expressivity, and gene–environment interactions. Thus, mtDNA genetics contributes to the quantitative and environmental components of human genetics that cannot be explained by Mendelian genetics. Because mtDNA is maternally inherited and cytoplasmic, it has fostered the first germline gene therapy, nuclear transplantation. However, effective interventions are still lacking for existing patients with mitochondrial dysfunction.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Wallace, D. C. A mitochondrial bioenergetic etiology of disease. J. Clin. Invest. 123, 1405–1412 (2013).
Pham, T. D. et al. Cristae remodeling causes acidification detected by integrated graphene sensor during mitochondrial outer membrane permeabilization. Sci. Rep. 6, 35907 (2016).
Walker, J. E. The ATP synthase: the understood, the uncertain and the unknown. Biochem. Soc. Trans. 41, 1–16 (2013).
Wallace, D. C. & Fan, W. Energetics, epigenetics, mitochondrial genetics. Mitochondrion 10, 12–31 (2010).
Wallace, D. C., Fan, W. & Procaccio, V. Mitochondrial energetics and therapeutics. Annu. Rev. Pathol. 5, 297–348 (2010).
Picard, M. et al. Progressive increase in mtDNA 3243A>G heteroplasmy causes abrupt transcriptional reprogramming. Proc. Natl. Acad. Sci. USA 111, E4033–E4042 (2014).
Couvillion, M. T., Soto, I. C., Shipkovenska, G. & Churchman, L. S. Synchronized mitochondrial and cytosolic translation programs. Nature 533, 499–503 (2016).
Giles, R. E., Blanc, H., Cann, H. M. & Wallace, D. C. Maternal inheritance of human mitochondrial DNA. Proc. Natl. Acad. Sci. USA 77, 6715–6719 (1980).
Pyle, A. et al. Extreme-depth re-sequencing of mitochondrial DNA finds no evidence of paternal transmission in humans. PLoS Genet. 11, e1005040 (2015).
Brown, W. M., Prager, E. M., Wang, A. & Wilson, A. C. Mitochondrial DNA sequences of primates: tempo and mode of evolution. J. Mol. Evol. 18, 225–239 (1982).
Neckelmann, N., Li, K., Wade, R. P., Shuster, R. & Wallace, D. C. cDNA sequence of a human skeletal muscle ADP/ATP translocator: lack of a leader peptide, divergence from a fibroblast translocator cDNA, and coevolution with mitochondrial DNA genes. Proc. Natl. Acad. Sci. USA 84, 7580–7584 (1987).
Wallace, D. C. et al. Sequence analysis of cDNAs for the human and bovine ATP synthase beta subunit: mitochondrial DNA genes sustain seventeen times more mutations. Curr. Genet. 12, 81–90 (1987).
Wallace, D. C. Mitochondrial DNA variation in human radiation and disease. Cell 163, 33–38 (2015).
Sorrentino, V., Menzies, K. J. & Auwerx, J. Repairing mitochondrial dysfunction in disease. Annu. Rev. Pharmacol. Toxicol. 58, 353–389 (2018).
Gorman, G. S. et al. Prevalence of nuclear and mitochondrial DNA mutations related to adult mitochondrial disease. Ann. Neurol. 77, 753–759 (2015).
Lightowlers, R. N., Taylor, R. W. & Turnbull, D. M. Mutations causing mitochondrial disease: what is new and what challenges remain? Science 349, 1494–1499 (2015).
Elliott, H. R., Samuels, D. C., Eden, J. A., Relton, C. L. & Chinnery, P. F. Pathogenic mitochondrial DNA mutations are common in the general population. Am. J. Hum. Genet. 83, 254–260 (2008).
Chinnery, P. F., Elliott, H. R., Hudson, G., Samuels, D. C. & Relton, C. L. Epigenetics, epidemiology and mitochondrial DNA diseases. Int. J. Epidemiol. 41, 177–187 (2012).
Rebolledo-Jaramillo, B. et al. Maternal age effect and severe germ-line bottleneck in the inheritance of human mitochondrial DNA. Proc. Natl. Acad. Sci. USA 111, 15474–15479 (2014).
Wallace, D. C. et al. Mitochondrial DNA mutation associated with Leber’s hereditary optic neuropathy. Science 242, 1427–1430 (1988).
Holt, I. J., Harding, A. E., Petty, R. K. & Morgan-Hughes, J. A. A new mitochondrial disease associated with mitochondrial DNA heteroplasmy. Am. J. Hum. Genet. 46, 428–433 (1990).
Wallace, D. C. et al. Familial mitochondrial encephalomyopathy (MERRF): genetic, pathophysiological, and biochemical characterization of a mitochondrial DNA disease. Cell 55, 601–610 (1988).
Shoffner, J. M. et al. Myoclonic epilepsy and ragged-red fiber disease (MERRF) is associated with a mitochondrial DNA tRNA(Lys) mutation. Cell 61, 931–937 (1990).
Goto, Y., Nonaka, I. & Horai, S. A mutation in the tRNA(Leu)(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature 348, 651–653 (1990).
Huoponen, K., Vilkki, J., Aula, P., Nikoskelainen, E. K. & Savontaus, M. L. A new mtDNA mutation associated with Leber hereditary optic neuroretinopathy. Am. J. Hum. Genet. 48, 1147–1153 (1991).
Johns, D. R., Neufeld, M. J. & Park, R. D. An ND-6 mitochondrial DNA mutation associated with Leber hereditary optic neuropathy. Biochem. Biophys. Res. Commun. 187, 1551–1557 (1992).
Fischel-Ghodsian, N., Prezant, T. R., Bu, X. & Oztas, S. Mitochondrial ribosomal RNA gene mutation in a patient with sporadic aminoglycoside ototoxicity. Am. J. Otolaryngol. 14, 399–403 (1993).
Hutchin, T. et al. A molecular basis for human hypersensitivity to aminoglycoside antibiotics. Nucleic Acids Res. 21, 4174–4179 (1993).
Pfeffer, G. & Chinnery, P. F. Diagnosis and treatment of mitochondrial myopathies. Ann. Med. 45, 4–16 (2013).
Andreu, A. L. et al. A nonsense mutation (G15059A) in the cytochrome b gene in a patient with exercise intolerance and myoglobinuria. Ann. Neurol. 45, 127–130 (1999).
Andreu, A. L. et al. Exercise intolerance due to mutations in the cytochrome b gene of mitochondrial DNA. N. Engl. J. Med. 341, 1037–1044 (1999).
Andreu, A. L., Checcarelli, N., Iwata, S., Shanske, S. & DiMauro, S. A missense mutation in the mitochondrial cytochrome b gene in a revisited case with histiocytoid cardiomyopathy. Pediatr. Res. 48, 311–314 (2000).
Mancuso, M. et al. Mitochondrial myopathy and complex III deficiency in a patient with a new stop-codon mutation (G339X) in the cytochrome b gene. J. Neurol. Sci. 209, 61–63 (2003).
DiMauro, S. Mitochondrial encephalomyopathies—at fifty years on: the Robert Wartenberg lecture. Neurology 81, 281–291 (2013).
Emmanuele, V. et al. A novel mutation in the mitochondrial DNA cytochrome b gene (MTCYB) in a patient with mitochondrial encephalomyopathy, lactic acidosis, and strokelike episodes syndrome. J. Child Neurol. 28, 236–242 (2013).
Wallace, D.C., Lott, M.T. & Procaccio, V. Mitochondrial medicine: the mitochondrial biology and genetics of metabolic and degenerative diseases, cancer, and aging. in Emery and Rimoin’s Principles and Practice of Medical Genetics Vol. 1 (eds. Rimoin, D. L. et al.) Ch. 11, 1–153 (Churchill Livingstone Elsevier, Philadelphia, 2013).
Wallace, D.C., Lott, M.T. & Procaccio, V. Mitochondrial biology—mitochondrial medicine. in Principles and Practice of Medical Genetics and Genomics (eds. Pyeritz, R. E. et al.) (Elsevier, Philadelphia, 2018).
MITOMAP. A Human Mitochondrial Genome Database. http://www.mitomap.org/ (2018).
Jenuth, J. P., Peterson, A. C., Fu, K. & Shoubridge, E. A. Random genetic drift in the female germline explains the rapid segregation of mammalian mitochondrial DNA. Nat. Genet. 14, 146–151 (1996).
Wai, T., Teoli, D. & Shoubridge, E. A. The mitochondrial DNA genetic bottleneck results from replication of a subpopulation of genomes. Nat. Genet. 40, 1484–1488 (2008).
Cree, L. M. et al. A reduction of mitochondrial DNA molecules during embryogenesis explains the rapid segregation of genotypes. Nat. Genet. 40, 249–254 (2008).
Li, M. et al. Transmission of human mtDNA heteroplasmy in the genome of the Netherlands families: support for a variable-size bottleneck. Genome Res. 26, 417–426 (2016).
Wilson, I. J. et al. Mitochondrial DNA sequence characteristics modulate the size of the genetic bottleneck. Hum. Mol. Genet. 25, 1031–1041 (2016).
Carling, P. J., Cree, L. M. & Chinnery, P. F. The implications of mitochondrial DNA copy number regulation during embryogenesis. Mitochondrion 11, 686–692 (2011).
Jokinen, R. & Battersby, B. J. Insight into mammalian mitochondrial DNA segregation. Ann. Med. 45, 149–155 (2013).
Floros, V. I. et al. Segregation of mitochondrial DNA heteroplasmy through a developmental genetic bottleneck in human embryos. Nat. Cell Biol. 20, 144–151 (2018).
Fan, W. et al. A mouse model of mitochondrial disease reveals germline selection against severe mtDNA mutations. Science 319, 958–962 (2008).
Stewart, J. B. et al. Strong purifying selection in transmission of mammalian mitochondrial DNA. PLoS Biol. 6, e10 (2008).
Sharpley, M. S. et al. Heteroplasmy of mouse mtDNA is genetically unstable and results in altered behavior and cognition. Cell 151, 333–343 (2012).
Shoffner, J. M. et al. Mitochondrial DNA variants observed in Alzheimer disease and Parkinson disease patients. Genomics 17, 171–184 (1993).
Hutchin, T. & Cortopassi, G. A mitochondrial DNA clone is associated with increased risk for Alzheimer disease. Proc. Natl. Acad. Sci. USA 92, 6892–6895 (1995).
Santoro, A. et al. Evidence for sub-haplogroup h5 of mitochondrial DNA as a risk factor for late onset Alzheimer’s disease. PLoS One 5, e12037 (2010).
Chagnon, P. et al. Phylogenetic analysis of the mitochondrial genome indicates significant differences between patients with Alzheimer disease and controls in a French-Canadian founder population. Am. J. Med. Genet. 85, 20–30 (1999).
Carrieri, G. et al. Mitochondrial DNA haplogroups and APOE4 allele are non-independent variables in sporadic Alzheimer’s disease. Hum. Genet. 108, 194–198 (2001).
van der Walt, J. M. et al. Analysis of European mitochondrial haplogroups with Alzheimer disease risk. Neurosci. Lett. 365, 28–32 (2004).
Lakatos, A. et al. Association between mitochondrial DNA variations and Alzheimer’s disease in the ADNI cohort. Neurobiol. Aging 31, 1355–1363 (2010).
van der Walt, J. M. et al. Mitochondrial polymorphisms significantly reduce the risk of Parkinson disease. Am. J. Hum. Genet. 72, 804–811 (2003).
Ghezzi, D. et al. Mitochondrial DNA haplogroup K is associated with a lower risk of Parkinson’s disease in Italians. Eur. J. Hum. Genet. 13, 748–752 (2005).
Khusnutdinova, E. et al. A mitochondrial etiology of neurodegenerative diseases: evidence from Parkinson’s disease. Ann. NY Acad. Sci. 1147, 1–20 (2008).
Fachal, L. et al. No evidence of association between common European mitochondrial DNA variants in Alzheimer, Parkinson, and migraine in the Spanish population. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 168B, 54–65 (2015).
Hudson, G. et al. Two-stage association study and meta-analysis of mitochondrial DNA variants in Parkinson disease. Neurology 80, 2042–2048 (2013).
Chalkia, D. et al. Association between mitochondrial DNA haplogroup variation and autism spectrum disorders. JAMA Psychiatry 74, 1161–1168 (2017).
Wang, Y., Picard, M. & Gu, Z. Genetic evidence for elevated pathogenicity of mitochondrial DNA heteroplasmy in Autism Spectrum Disorder. PLoS Genet. 12, e1006391 (2016).
Fuku, N. et al. Mitochondrial haplogroup N9a confers resistance against type 2 diabetes in Asians. Am. J. Hum. Genet. 80, 407–415 (2007).
Nishigaki, Y. et al. Mitochondrial haplogroup N9b is protective against myocardial infarction in Japanese males. Hum. Genet. 120, 827–836 (2007).
Chalkia, D. et al. Mitochondrial DNA associations with East Asian metabolic syndrome. Biochim. Biophys. Acta 1859, 878–892 (2018).
Latorre-Pellicer, A. et al. Mitochondrial and nuclear DNA matching shapes metabolism and healthy ageing. Nature 535, 561–565 (2016).
Yu, X. et al. Dissecting the effects of mtDNA variations on complex traits using mouse conplastic strains. Genome Res. 19, 159–165 (2009).
Wallace, D. C. Genetics: mitochondrial DNA in evolution and disease. Nature 535, 498–500 (2016).
Wallace, D. C. & Chalkia, D. Mitochondrial DNA genetics and the heteroplasmy conundrum in evolution and disease. Cold Spring Harb. Perspect. Biol. 5, a021220 (2013).
Craven, L. et al. Pronuclear transfer in human embryos to prevent transmission of mitochondrial DNA disease. Nature 465, 82–85 (2010).
Hyslop, L. A. et al. Towards clinical application of pronuclear transfer to prevent mitochondrial DNA disease. Nature 534, 383–386 (2016).
Tachibana, M. et al. Mitochondrial gene replacement in primate offspring and embryonic stem cells. Nature 461, 367–372 (2009).
Tachibana, M. et al. Towards germline gene therapy of inherited mitochondrial diseases. Nature 493, 627–631 (2013).
Kang, E. et al. Mitochondrial replacement in human oocytes carrying pathogenic mitochondrial DNA mutations. Nature 540, 270–275 (2016).
Paull, D. et al. Nuclear genome transfer in human oocytes eliminates mitochondrial DNA variants. Nature 493, 632–637 (2013).
Wolf, D. P., Hayama, T. & Mitalipov, S. Mitochondrial genome inheritance and replacement in the human germline. EMBO J. 36, 2177–2181 (2017).
Ma, H. et al. Functional human oocytes generated by transfer of polar body genomes. Cell Stem Cell 20, 112–119 (2017).
Slone, J., Zhang, J. & Huang, T. Experience from the first live-birth derived from oocyte nuclear transfer as a treatment strategy for mitochondrial diseases. J. Mol. Genet. Med. 11, 1000258 (2017).
Yamada, M. et al. Genetic drift can compromise mitochondrial replacement by nuclear transfer in human oocytes. Cell Stem Cell 18, 749–754 (2016).
Barritt, J. A., Brenner, C. A., Malter, H. E. & Cohen, J. Mitochondria in human offspring derived from ooplasmic transplantation. Hum. Reprod. 16, 513–516 (2001).
Zhang, J. et al. Live birth derived from oocyte spindle transfer to prevent mitochondrial disease. Reprod. Biomed. Online 34, 361–368 (2017).
Kujoth, G. C. et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 309, 481–484 (2005).
Trifunovic, A. et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429, 417–423 (2004).
Vermulst, M. et al. DNA deletions and clonal mutations drive premature aging in mitochondrial mutator mice. Nat. Genet. 40, 392–394 (2008).
Kang, E. et al. Age-related accumulation of somatic mitochondrial DNA mutations in adult-derived human iPSCs. Cell Stem Cell 18, 625–636 (2016).
Schriner, S. E. et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science 308, 1909–1911 (2005).
Lee, H. Y. et al. Targeted expression of catalase to mitochondria prevents age-associated reductions in mitochondrial function and insulin resistance. Cell Metab. 12, 668–674 (2010).
Woo, D. K. et al. Mitochondrial genome instability and ROS enhance intestinal tumorigenesis in APC(Min/+) mice. Am. J. Pathol. 180, 24–31 (2012).
Arnheim, N. & Cortopassi, G. Deleterious mitochondrial DNA mutations accumulate in aging human tissues. Mutat. Res. 275, 157–167 (1992).
Soong, N. W., Hinton, D. R., Cortopassi, G. & Arnheim, N. Mosaicism for a specific somatic mitochondrial DNA mutation in adult human brain. Nat. Genet. 2, 318–323 (1992).
Corral-Debrinski, M. et al. Mitochondrial DNA deletions in human brain: regional variability and increase with advanced age. Nat. Genet. 2, 324–329 (1992).
Corral-Debrinski, M. et al. Marked changes in mitochondrial DNA deletion levels in Alzheimer brains. Genomics 23, 471–476 (1994).
Coskun, P. E., Beal, M. F. & Wallace, D. C. Alzheimer’s brains harbor somatic mtDNA control-region mutations that suppress mitochondrial transcription and replication. Proc. Natl. Acad. Sci. USA 101, 10726–10731 (2004).
Coskun, P. E. et al. Systemic mitochondrial dysfunction and the etiology of Alzheimer’s disease and down syndrome dementia. J. Alzheimers Dis. 20(Suppl 2), S293–S310 (2010).
Coskun, P. et al. A mitochondrial etiology of Alzheimer and Parkinson disease. Biochim. Biophys. Acta 1820, 553–564 (2012).
Horton, T. M. et al. Marked increase in mitochondrial DNA deletion levels in the cerebral cortex of Huntington’s disease patients. Neurology 45, 1879–1883 (1995).
Corral-Debrinski, M. et al. Hypoxemia is associated with mitochondrial DNA damage and gene induction. Implications for cardiac disease. J. Am. Med. Assoc. 266, 1812–1816 (1991).
Ji, F. et al. Mitochondrial DNA variant associated with Leber hereditary optic neuropathy and high-altitude Tibetans. Proc. Natl. Acad. Sci. USA 109, 7391–7396 (2012).
Zheng, S. et al. Role of mtDNA haplogroups in COPD susceptibility in a southwestern Han Chinese population. Free Radic. Biol. Med. 53, 473–481 (2012).
Sherer, T. B. et al. Mechanism of toxicity of pesticides acting at complex I: relevance to environmental etiologies of Parkinson’s disease. J. Neurochem. 100, 1469–1479 (2007).
Cannon, J. R. & Greenamyre, J. T. Neurotoxic in vivo models of Parkinson’s disease recent advances. Prog. Brain Res. 184, 17–33 (2010).
Bulstrode, H. et al. Mitochondrial DNA and traumatic brain injury. Ann. Neurol. 75, 186–195 (2014).
Kilbaugh, T. J. et al. Peripheral blood mitochondrial DNA as a biomarker of cerebral mitochondrial dysfunction following traumatic brain injury in a porcine model. PLoS One 10, e0130927 (2015).
Baudouin, S. V. et al. Mitochondrial DNA and survival after sepsis: a prospective study. Lancet 366, 2118–2121 (2005).
Gomez, R. et al. Association of mitochondrial allele 4216C with increased risk for complicated sepsis and death after traumatic injury. J. Trauma 66, 850–857 (2009). discussion 857–858.
Hendrickson, S. L. et al. Mitochondrial DNA haplogroups influence. AIDS progression. AIDS 22, 2429–2439 (2008).
Zhang, Q. et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464, 104–107 (2010).
Zhang, Q., Itagaki, K. & Hauser, C. J. Mitochondrial DNA is released by shock and activates neutrophils via p38 map kinase. Shock 34, 55–59 (2010).
Krysko, D. V. et al. Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends Immunol. 32, 157–164 (2011).
Dan Dunn, J., Alvarez, L. A., Zhang, X. & Soldati, T. Reactive oxygen species and mitochondria: A nexus of cellular homeostasis. Redox Biol. 6, 472–485 (2015).
Nadeau-Vallée, M. et al. A critical role of interleukin-1 in preterm labor. Cytokine Growth Factor Rev. 28, 37–51 (2016).
Angelin, A. et al. Foxp3 reprograms T cell metabolism to function in low-glucose, high-lactate environments. Cell Metab. 25, 1282–1293.e7 (2017).
Ravi, S., Mitchell, T., Kramer, P., Chacko, B. & Darley-Usmar, V. M. Mitochondria in monocytes and macrophages-implications for translational and basic research. Int. J. Biochem. Cell Biol. 53, 202–207 (2014).
Acknowledgements
This work was supported by NIH grants 5R01-NS021328-30, 1R01MH108592-01A1, 1R01MN110185-01A1, R01OD010944-05, and DOD W81XWH-16-1-0401 awarded to D.C.W.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wallace, D.C. Mitochondrial genetic medicine. Nat Genet 50, 1642–1649 (2018). https://doi.org/10.1038/s41588-018-0264-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41588-018-0264-z
This article is cited by
-
Leveraging new methods for comprehensive characterization of mitochondrial DNA in esophageal squamous cell carcinoma
Genome Medicine (2024)
-
Rapid genomic sequencing for genetic disease diagnosis and therapy in intensive care units: a review
npj Genomic Medicine (2024)
-
Induced pluripotent stem cells: ex vivo models for human diseases due to mitochondrial DNA mutations
Journal of Biomedical Science (2023)
-
A small protein coded within the mitochondrial canonical gene nd4 regulates mitochondrial bioenergetics
BMC Biology (2023)
-
Mitochondrial heteroplasmic shifts reveal a positive selection of breast cancer
Journal of Translational Medicine (2023)