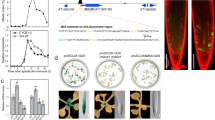
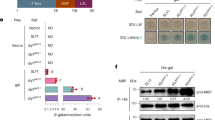
Abstract
Precise control of plant stem cell proliferation is necessary for the continuous and reproducible development of plant organs1,2. The peptide ligand CLAVATA3 (CLV3) and its receptor protein kinase CLAVATA1 (CLV1) maintain stem cell homeostasis within a deeply conserved negative feedback circuit1,2. In Arabidopsis, CLV1 paralogs also contribute to homeostasis, by compensating for the loss of CLV1 through transcriptional upregulation3. Here, we show that compensation4,5 operates in diverse lineages for both ligands and receptors, but while the core CLV signaling module is conserved, compensation mechanisms have diversified. Transcriptional compensation between ligand paralogs operates in tomato, facilitated by an ancient gene duplication that impacted the domestication of fruit size. In contrast, we found little evidence for transcriptional compensation between ligands in Arabidopsis and maize, and receptor compensation differs between tomato and Arabidopsis. Our findings show that compensation among ligand and receptor paralogs is critical for stem cell homeostasis, but that diverse genetic mechanisms buffer conserved developmental programs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Raw data for all quantifications are included as Supplementary Tables. All RNA-seq data from tomato are available from the National Center for Biotechnology Information. The tomato Sequence Read Archive (SRA) project and BioProject accession nos. are SRP161864 and PRJNA491365, respectively. The maize SRA projects and BioProject accessions numbers are SRR7970748, SRR7970747, SRR7970749, SRR7970750 and PRJNA494874, respectively. RNA-seq data from Arabidopsis was obtained from Klepikova et al.54 and Mandel et al.55.
References
Somssich, M., Je, B. I., Simon, R. & Jackson, D. CLAVATA-WUSCHEL signaling in the shoot meristem. Development 143, 3238–3248 (2016).
Soyars, C. L., James, S. R. & Nimchuk, Z. L. Ready, aim, shoot: stem cell regulation of the shoot apical meristem. Curr. Opin. Plant Biol. 29, 163–168 (2016).
Nimchuk, Z. L., Zhou, Y., Tarr, P. T., Peterson, B. A. & Meyerowitz, E. M. Plant stem cell maintenance by transcriptional cross-regulation of related receptor kinases. Development 142, 1043–1049 (2015).
Diss, G., Ascencio, D., DeLuna, A. & Landry, C. R. Molecular mechanisms of paralogous compensation and the robustness of cellular networks. J. Exp. Zool. B Mol. Dev. Evol. 322, 488–499 (2014).
El-Brolosy, M. A. & Stainier, D. Y. R. Genetic compensation: a phenomenon in search of mechanisms. PLoS Genet. 13, e1006780 (2017).
Xu, C. et al. A cascade of arabinosyltransferases controls shoot meristem size in tomato. Nat. Genet. 47, 784–792 (2015).
Goad, D. M., Zhu, C. & Kellogg, E. A. Comprehensive identification and clustering of CLV3/ESR-related (CLE) genes in plants finds groups with potentially shared function. New Phytol. 216, 605–616 (2017).
Kafri, R., Bar-Even, A. & Pilpel, Y. Transcription control reprogramming in genetic backup circuits. Nat. Genet. 37, 295–299 (2005).
Kafri, R., Springer, M. & Pilpel, Y. Genetic redundancy: new tricks for old genes. Cell 136, 389–392 (2009).
Nimchuk, Z. L. CLAVATA1 controls distinct signaling outputs that buffer shoot stem cell proliferation through a two-step transcriptional compensation loop. PLoS Genet. 13, e1006681 (2017).
Yamaguchi, Y. L. et al. A collection of mutants for CLE-peptide-encoding genes in Arabidopsis generated by CRISPR/Cas9-mediated gene targeting. Plant Cell Physiol. 58, 1848–1856 (2017).
Gregory, E. F., Dao, T. Q., Alexander, M. A., Miller, M. J. & Fletcher, J. C. The signaling peptide-encoding genes CLE16, CLE17 and CLE27 are dispensable for Arabidopsis shoot apical meristem activity. PLoS ONE 13, e0202595 (2018).
Betsuyaku, S., Sawa, S. & Yamada, M. The function of the CLE peptides in plant development and plant-microbe interactions. Arabidopsis Book 9, e0149 (2011).
Clark, S. E., Running, M. P. & Meyerowitz, E. M. CLAVATA3 is a specific regulator of shoot and floral meristem development affecting the same processes as CLAVATA1. Development 121, 2057–2067 (1995).
Kayes, J. M. & Clark, S. E. CLAVATA2, a regulator of meristem and organ development in Arabidopsis. Development 125, 3843–3851 (1998).
Li, H. et al. Evolution of the leucine‐rich repeat receptor‐like protein kinase gene family: ancestral copy number and functional divergence of BAM1 and BAM2 in Brassicaceae. J. Syst. Evol. 54, 204–218 (2016).
Deyoung, B. J. & Clark, S. E. BAM receptors regulate stem cell specification and organ development through complex interactions with CLAVATA signaling. Genetics 180, 895–904 (2008).
Bombarely, A. et al. Insight into the evolution of the Solanaceae from the parental genomes of Petunia hybrida. Nat. Plants 2, 16074 (2016).
Hoshino, A. et al. Genome sequence and analysis of the Japanese morning glory Ipomoea nil. Nat. Commun. 7, 13295 (2016).
Freeling, M. et al. Fractionation mutagenesis and similar consequences of mechanisms removing dispensable or less-expressed DNA in plants. Curr. Opin. Plant Biol. 15, 131–139 (2012).
Kumar, S., Stecher, G., Suleski, M. & Hedges, S. B. TimeTree: a resource for timelines, timetrees, and divergence times. Mol. Biol. Evol. 34, 1812–1819 (2017).
Suzaki, T., Yoshida, A. & Hirano, H.-Y. Functional diversification of CLAVATA3-related CLE proteins in meristem maintenance in rice. Plant Cell 20, 2049–2058 (2008).
Je, B. I. et al. Signaling from maize organ primordia via FASCIATED EAR3 regulates stem cell proliferation and yield traits. Nat. Genet. 48, 785–791 (2016).
Abley, K., Locke, J. C. W. & Leyser, H. M. O. Developmental mechanisms underlying variable, invariant and plastic phenotypes. Ann. Bot. 117, 733–748 (2016).
Bernier, G. The control of floral evocation and morphogenesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 39, 175–219 (1988).
Depuydt, S. et al. Suppression of Arabidopsis protophloem differentiation and root meristem growth by CLE45 requires the receptor-like kinase BAM3. Proc. Natl Acad. Sci. USA 110, 7074–7079 (2013).
Kafri, R., Levy, M. & Pilpel, Y. The regulatory utilization of genetic redundancy through responsive backup circuits. Proc. Natl Acad. Sci. USA 103, 11653–11658 (2006).
Soria, P. S., McGary, K. L. & Rokas, A. Functional divergence for every paralog. Mol. Biol. Evol. 31, 984–992 (2014).
Müller, R., Borghi, L., Kwiatkowska, D., Laufs, P. & Simon, R. Dynamic and compensatory responses of Arabidopsis shoot and floral meristems to CLV3 signaling. Plant Cell 18, 1188–1198 (2006).
Fletcher, J. C. The CLV-WUS stem cell signaling pathway: a roadmap to crop yield optimization. Plants (Basel) 7, E87 (2018).
Rodríguez-Leal, D., Lemmon, Z. H., Man, J., Bartlett, M. E. & Lippman, Z. B. Engineering quantitative trait variation for crop improvement by genome editing. Cell 171, 470–480.e8 (2017).
Park, S. J., Jiang, K., Schatz, M. C. & Lippman, Z. B. Rate of meristem maturation determines inflorescence architecture in tomato. Proc. Natl Acad. Sci. USA 109, 639–644 (2012).
Brooks, C., Nekrasov, V., Lippman, Z. B. & Van Eck, J. Efficient gene editing in tomato in the first generation using the clustered regularly interspaced short palindromic repeats/CRISPR-associated9 system. Plant Physiol. 166, 1292–1297 (2014).
Werner, S., Engler, C., Weber, E., Gruetzner, R. & Marillonnet, S. Fast track assembly of multigene constructs using Golden Gate cloning and the MoClo system. Bioeng. Bugs 3, 38–43 (2012).
Gupta, S. & Van Eck, J. Modification of plant regeneration medium decreases the time for recovery of Solanum lycopersicum cultivar M82 stable transgenic lines. Plant Cell Tissue Organ Cult. 127, 417–423 (2016).
Peterson, B. A. et al. Genome-wide assessment of efficiency and specificity in CRISPR/Cas9 mediated multiple site targeting in Arabidopsis. PLoS ONE 11, e0162169 (2016).
Char, S. N. et al. An Agrobacterium-delivered CRISPR/Cas9 system for high-frequency targeted mutagenesis in maize. Plant Biotechnol. J. 15, 257–268 (2017).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Sato, S. et al. The tomato genome sequence provides insights into fleshy fruit evolution. Nature 485, 635–641 (2012).
Kim, D. et al. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 14, R36 (2013).
Li, H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Anders, S., Pyl, P. T. & Huber, W. HTSeq: a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015).
Schnable, P. S. et al. The B73 maize genome: complexity, diversity, and dynamics. Science 326, 1112–1115 (2009).
R Core Team. R: a language and environment for statistical computing (R Foundation for Statistical Computing, 2015).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Lemmon, Z. H. et al. The evolution of inflorescence diversity in the nightshades and heterochrony during meristem maturation. Genome Res. 26, 1676–1686 (2016).
Nakagawa, T. et al. Improved Gateway binary vectors: high-performance vectors for creation of fusion constructs in transgenic analysis of plants. Biosci. Biotechnol. Biochem. 71, 2095–2100 (2007).
Zimmermann, L. et al. A completely reimplemented MPI Bioinformatics Toolkit with a new HHpred server at its core. J. Mol. Biol. 430, 2237–2243 (2018).
Frickey, T. & Lupas, A. CLANS: a Java application for visualizing protein families based on pairwise similarity. Bioinformatics 20, 3702–3704 (2004).
Lyons, E. et al. Finding and comparing syntenic regions among Arabidopsis and the outgroups papaya, poplar, and grape: CoGe with rosids. Plant Physiol. 148, 1772–1781 (2008).
Lemmon, Z. H. et al. Rapid improvement of domestication traits in an orphan crop by genome editing. Nat. Plants 4, 766–770 (2018).
Katoh, K. & Standley, D. M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780 (2013).
Besemer, J. & Borodovsky, M. GeneMark: web software for gene finding in prokaryotes, eukaryotes and viruses. Nucleic Acids Res. 33, W451–W454 (2005).
Klepikova, A. V., Logacheva, M. D., Dmitriev, S. E. & Penin, A. A. RNA-seq analysis of an apical meristem time series reveals a critical point in Arabidopsis thaliana flower initiation. BMC Genomics 16, 466 (2015).
Mandel, T. et al. Differential regulation of meristem size, morphology and organization by the ERECTA, CLAVATA and class III HD-ZIP pathways. Development 143, 1612–1622 (2016).
Acknowledgements
We thank C. Brooks, A. Krainer and J. Dalrymple for their technical support; P. Keen for assistance with the tomato transformation; T. Mulligan, S. Vermylen and S. Qiao from the Cold Spring Harbor Laboratory, and staff from Cornell University’s Long Island Horticultural Research and Extension Center, for assistance with plant care; and H. Shinohara and Y. Matsubayashi from Nagoya University for the tomato peptide binding assays. Initial work from Z.L.N. was supported by funds from the Virginia Polytechnic Institute and State University. The research for this study was supported by: a PEW Latin American Fellowship (no. 29661) to D.R.L.; a NIGMS MIRA award from the National Institutes of Health to Z.L.N. (award no. R35GM119614-01); an Agriculture and Food Research Initiative competitive grant no. 2016-67013-24572 of the USDA National Institute of Food and Agriculture and the Next-Generation BioGreen 21 Program SSAC (grant no. PJ01322602) from the Rural Development Administration, Republic of Korea to D.J.; an Agriculture and Food Research Initiative competitive grant no. 2015-67013-22823 of the USDA National Institute of Food and Agriculture to Z.B.L.; and the National Science Foundation Plant Genome Research Program (grant no. IOS-1732253 to J.V.E. and Z.B.L., and grant no. IOS-1546837 to D.J., M.E.B., Z.L.N and Z.B.L.).
Author information
Authors and Affiliations
Contributions
These authors contributed equally: C.-T.K., C.S., E.D.A. and J.M. D.R.L. performed the tomato CRISPR and Arabidopsis molecular and phenotypic analyses, prepared the figures and wrote the manuscript. C.X. performed the tomato CRISPR and complementation experiments, genetic and phenotypic analyses, prepared the figures and helped edit the manuscript. C.T.K. performed the tomato CRISPR experiments, and the genetic, RNA-seq and phenotypic analyses. C.S. generated the Arabidopsis CRISPR knockouts, performed the plant transformations and the genetic and phenotypic analyses. E.D.A. performed the maize CRISPR experiments and the genetic and phenotypic analyses. L.L. performed the maize RNA-seq analyses. J.M. performed the synteny and CLE clustering analyses. Z.H.L. performed the tomato RNA-seq analyses. D.S.J. performed the Arabidopsis genetic and phenotypic analyses. J.V.E. performed the tomato transformations. D.P.J. conceived and supervised the maize research and edited the manuscript. M.E.B. conceived, designed and guided the synteny and CLE clustering experiments and helped prepare the figures and write the manuscript. Z.L.N. conceived and supervised the Arabidopsis research and wrote and edited the manuscript. Z.B.L. conceived and led the research, supervised and performed the experiments, and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Fig. 1 CRISPR–Cas9 targeting and genetic analysis of SlCLE3.
a, CRISPR/Cas9 design and sequencing of targeted regions in SlCLV3, SlCLE9 and SlCLE3. Black boxes indicate exons. Red arrows, single-guide RNAs (gRNAs; Target 1 and Target 2). Black arrows, forward (F) and reverse (R) primers for PCR genotyping and sequencing. Sequences of slclv3, slcle9 and slcle3 mutant alleles 1 and 2 (a1 and a2) identified from T0 plants. gRNA target sequences in red, and protospacer adjacent motif sequences in bold and underlined. Blue dashed lines, deletions. Space between each target region is indicated in parentheses. b, Representative inflorescences from axillary shoots in slclv3, slclv3 slcle9, slclv3 slcle9 slcle3 and slcle9 slcle3 mutants. Red arrowheads, branches. c, RT–qPCR of SlCLE3 from reproductive meristems of WT and slclv3 normalized to SlUBI. Mean ± s.e.m.; two biological and three technical replicates). d, Quantification of locule number in slcle9 and slcle9 slcle3, slclv3 slcle9 and slclv3 slcle9 slcle3 (n = 166, 31, 34 and 28). Boxplots, 25th–75th percentile; whiskers, full data range; center line, median. One-way ANOVA and Tukey; letters represent significance groups at P < 0.05 in d. Scale bar, 2 cm in c.
Supplementary Fig. 2 Expression analysis and CRISPR–Cas9 multiplex targeting of Arabidopsis CLE gene family members.
a, RNA-seq data of all CLE family members in WT meristems from 7 to 16 d after germination (DAG). Expression levels represented as total gene reads normalized by size factor for each CLE gene. Data are from Klepikova et al.54. b, RNA-seq data of all Arabidopsis CLE family members in single biological replicates at 8 and 10 DAG from WT and clv3. Expression levels are shown as reads per kilobase of transcript per million mapped reads. Data are from Mandel et al.55. c, CRISPR/Cas9 generated mutations in 11 Arabidopsis CLE genes in the clv3 background. In red, gRNA target sequences. Protospacer adjacent motif sequences are bold and underlined. Space between each target region is indicated in parenthesis. Blue dashed lines, deletions. Blue letters indicate nucleotide insertions compared to WT. Asteriks, in-frame mutations. The in-frame mutation within CLE11 removes the first three amino acids of the dodecapeptide and is therefore likely a null allele. The second mutation in CLE19 restores frame after an intervening mutant sequence of more than 25 amino acids.
Supplementary Fig. 3 Arabidopsis passive CLE compensation is mediated by CLV1 and BAM receptors.
a,b, Representative confocal micrographs and meristem quantifications (perimeter, height, width) of WT, clv1, clv3, clv1 clv3, bam1/2/3, clv3 bam1/2/3 and clv1 bam1/2/3 (n = 16, 15, 15, 15, 15, 15, 11, and 13). c, Carpel number quantifications of same genotypes as in b, (n = 150, 116, 150, 150, 150, 144, 77, 50). One-way ANOVA and Tukey test; letters represent significance groups at P < 0.05 in b and c. Scale bar, 50 µm in a.
Supplementary Fig. 4 CRISPR–Cas9 targeting and genetic analysis of the leucine‐rich repeat receptor genes in tomato.
a, CRISPR–Cas9 design and sequencing of targeted regions in SlCLV1, SlCLV2, SlBAM1 and SlBAM4. Black boxes, exons. Red arrows, gRNAs (Target 1 and Target 2). Black arrows, forward (F) and reverse (R) primers for PCR genotyping and sequencing. Sequences of T0 plants from CR-slclv1, CR-slclv2, CR-slbam1 and CR-slbam4 mutant alleles 1 and 2 (a1 and a2, respectively). gRNA target sequences in red, and protospacer-adjacent motif sequences in bold and underlined. Blue dashed lines, deletions. Space between each target region is indicated in parentheses. b, Representative inflorescences from single and higher-order mutants. Red arrowheads, branches. c, Fold change in expression of SlBAM1, SlBAM2, SlBAM3 and SlBAM4 relative to WT RNA-seq from single slclv1, slclv3 and double mutants slclv1 slclv3 and slcv3 slcle9 (two biological replicates, 4-fold change, 1 cpm cutoff, FDR < 0.10). d, Quantification of locule number from single and higher-order mutants (n = 104, 94, 92, 35, 43, 166, 77, 116, 58, 113, 38, 35, 63, 24 and 34). Blue dashed line, WT mean. Boxplots, 25th–75th percentile; whiskers, full data range; center line, median. One-way ANOVA and Tukey; letters represent significance groups at P < 0.05 in d. Scale bar, 2 cm in b.
Supplementary information
Supplementary Information
Supplementary Figures 1–4
Supplementary Tables 1–8
Supplementary Tables 1–8
Rights and permissions
About this article
Cite this article
Rodriguez-Leal, D., Xu, C., Kwon, CT. et al. Evolution of buffering in a genetic circuit controlling plant stem cell proliferation. Nat Genet 51, 786–792 (2019). https://doi.org/10.1038/s41588-019-0389-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41588-019-0389-8
This article is cited by
-
Multiple variation patterns of terpene synthases in 26 maize genomes
BMC Genomics (2023)
-
A network of CLAVATA receptors buffers auxin-dependent meristem maintenance
Nature Plants (2023)
-
SlCK2α as a novel substrate for CRL4 E3 ligase regulates fruit size through maintenance of cell division homeostasis in tomato
Planta (2023)
-
Novel insights into maize (Zea mays) development and organogenesis for agricultural optimization
Planta (2023)
-
Trait Improvement of Solanaceae Fruit Crops for Vertical Farming by Genome Editing
Journal of Plant Biology (2023)