Abstract
Numerous microRNAs and their target mRNAs are coexpressed across diverse cell types. However, it is unknown whether they are regulated in a manner independent of or dependent on cellular context. Here, we explored transcriptome-wide targeting and gene regulation by miR-155, whose activation-induced expression plays important roles in innate and adaptive immunity. Through mapping of miR-155 targets through differential iCLIP, mRNA quantification with RNA-seq, and 3′ untranslated region (UTR)-usage analysis with poly(A)-seq in macrophages, dendritic cells, and T and B lymphocytes either sufficient or deficient in activated miR-155, we identified numerous targets differentially bound by miR-155. Whereas alternative cleavage and polyadenylation (ApA) contributed to differential miR-155 binding to some transcripts, in most cases, identical 3′-UTR isoforms were differentially regulated across cell types, thus suggesting ApA-independent and cellular-context-dependent miR-155-mediated gene regulation. Our study provides comprehensive maps of miR-155 regulatory networks and offers a valuable resource for dissecting context-dependent and context-independent miRNA-mediated gene regulation in key immune cell types.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Xiao, C. & Rajewsky, K. MicroRNA control in the immune system: basic principles. Cell 136, 26–36 (2009).
O’Connell, R. M., Rao, D. S., Chaudhuri, A. A. & Baltimore, D. Physiological and pathological roles for microRNAs in the immune system. Nat. Rev. Immunol. 10, 111–122 (2010).
Bartel, D. P. MicroRNAs: target recognition and regulatory functions. Cell 136, 215–233 (2009).
Ameres, S. L. & Zamore, P. D. Diversifying microRNA sequence and function. Nat. Rev. Mol. Cell Biol. 14, 475–488 (2013).
Meister, G. Argonaute proteins: functional insights and emerging roles. Nat. Rev. Genet. 14, 447–459 (2013).
Loeb, G. B. et al. Transcriptome-wide miR-155 binding map reveals widespread noncanonical microRNA targeting. Mol. Cell 48, 760–770 (2012).
Chi, S. W., Hannon, G. J. & Darnell, R. B. An alternative mode of microRNA target recognition. Nat. Struct. Mol. Biol. 19, 321–327 (2012).
Chi, S. W., Zang, J. B., Mele, A. & Darnell, R. B. Argonaute HITS-CLIP decodes microRNA–mRNA interaction maps. Nature 460, 479–486 (2009).
Hafner, M. et al. Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell 141, 129–141 (2010).
Zisoulis, D. G. et al. Comprehensive discovery of endogenous Argonaute binding sites in Caenorhabditis elegans. Nat. Struct. Mol. Biol. 17, 173–179 (2010).
Farazi, T. A. et al. Identification of distinct miRNA target regulation between breast cancer molecular subtypes using AGO2-PAR-CLIP and patient datasets. Genome Biol. 15, R9 (2014).
Vigorito, E., Kohlhaas, S., Lu, D. & Leyland, R. miR-155: an ancient regulator of the immune system. Immunol. Rev. 253, 146–157 (2013).
Mashima, R. Physiological roles of miR-155. Immunology 145, 323–333 (2015).
Eis, P. S. et al. Accumulation of miR-155 and BIC RNA in human B cell lymphomas. Proc. Natl Acad. Sci. USA 102, 3627–3632 (2005).
Seddiki, N., Brezar, V., Ruffin, N., Lévy, Y. & Swaminathan, S. Role of miR-155 in the regulation of lymphocyte immune function and disease. Immunology 142, 32–38 (2014).
Lu, L. F. et al. A single miRNA-mRNA interaction affects the immune response in a context- and cell-type-specific manner. Immunity 43, 52–64 (2015).
Nam, J. W. et al. Global analyses of the effect of different cellular contexts on microRNA targeting. Mol. Cell 53, 1031–1043 (2014).
Erhard, F. et al. Widespread context dependency of microRNA-mediated regulation. Genome Res. 24, 906–919 (2014).
König, J. et al. iCLIP reveals the function of hnRNP particles in splicing at individual nucleotide resolution. Nat. Struct. Mol. Biol. 17, 909–915 (2010).
Derti, A. et al. A quantitative atlas of polyadenylation in five mammals. Genome Res. 22, 1173–1183 (2012).
Haasch, D. et al. T cell activation induces a noncoding RNA transcript sensitive to inhibition by immunosuppressant drugs and encoded by the proto-oncogene, BIC. Cell. Immunol. 217, 78–86 (2002).
van den Berg, A. et al. High expression of B-cell receptor inducible gene BIC in all subtypes of Hodgkin lymphoma. Genes Chromosom. Cancer 37, 20–28 (2003).
O’Connell, R. M., Taganov, K. D., Boldin, M. P., Cheng, G. & Baltimore, D. MicroRNA-155 is induced during the macrophage inflammatory response. Proc. Natl Acad. Sci. USA 104, 1604–1609 (2007).
Ceppi, M. et al. MicroRNA-155 modulates the interleukin-1 signaling pathway in activated human monocyte-derived dendritic cells. Proc. Natl Acad. Sci. USA 106, 2735–2740 (2009).
McCarthy, D. J., Chen, Y. & Smyth, G. K. Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res. 40, 4288–4297 (2012).
Robinson, M. D. & Oshlack, A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 11, R25 (2010).
Bartel, D. P. Metazoan microRNAs. Cell 173, 20–51 (2018).
Hausser, J., Syed, A. P., Bilen, B. & Zavolan, M. Analysis of CDS-located miRNA target sites suggests that they can effectively inhibit translation. Genome Res. 23, 604–615 (2013).
Grimson, A. et al. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol. Cell 27, 91–105 (2007).
Agarwal, V., Bell, G. W., Nam, J. W. & Bartel, D. P. Predicting effective microRNA target sites in mammalian mRNAs. eLife 4, e05005 (2015).
Helwak, A., Kudla, G., Dudnakova, T. & Tollervey, D. Mapping the human miRNA interactome by CLASH reveals frequent noncanonical binding. Cell 153, 654–665 (2013).
Grosswendt, S. et al. Unambiguous identification of miRNA:target site interactions by different types of ligation reactions. Mol. Cell 54, 1042–1054 (2014).
Broughton, J. P., Lovci, M. T., Huang, J. L., Yeo, G. W. & Pasquinelli, A. E. Pairing beyond the seed supports microRNA targeting specificity. Mol. Cell 64, 320–333 (2016).
Moore, M. J. et al. miRNA-target chimeras reveal miRNA 3′-end pairing as a major determinant of Argonaute target specificity. Nat. Commun. 6, 8864 (2015).
Salmena, L., Poliseno, L., Tay, Y., Kats, L. & Pandolfi, P. P. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell 146, 353–358 (2011).
Wang, Y. et al. Endogenous miRNA sponge lincRNA-RoR regulates Oct4, Nanog, and Sox2 in human embryonic stem cell self-renewal. Dev. Cell 25, 69–80 (2013).
Memczak, S. et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 495, 333–338 (2013).
Piwecka, M. et al. Loss of a mammalian circular RNA locus causes miRNA deregulation and affects brain function. Science 357, eaam8526 (2017).
Barrett, S. P. & Salzman, J. Circular RNAs: analysis, expression and potential functions. Development 143, 1838–1847 (2016).
Sandberg, R., Neilson, J. R., Sarma, A., Sharp, P. A. & Burge, C. B. Proliferating cells express mRNAs with shortened 3′ untranslated regions and fewer microRNA target sites. Science 320, 1643–1647 (2008).
Gruber, A. R. et al. Global 3′ UTR shortening has a limited effect on protein abundance in proliferating T cells. Nat. Commun. 5, 5465 (2014).
Anders, S., Reyes, A. & Huber, W. Detecting differential usage of exons from RNA-seq data. Genome Res. 22, 2008–2017 (2012).
Kramer, N. J. et al. Altered lymphopoiesis and immunodeficiency in miR-142 null mice. Blood 125, 3720–3730 (2015).
Mildner, A. et al. Mononuclear phagocyte miRNome analysis identifies miR-142 as critical regulator of murine dendritic cell homeostasis. Blood 121, 1016–1027 (2013).
Cho, S. et al. miR-23'~27'~24 clusters control effector T cell differentiation and function. J. Exp. Med. 213, 235–249 (2016).
Lee, T. I. & Young, R. A. Transcriptional regulation and its misregulation in disease. Cell 152, 1237–1251 (2013).
Bhattacharyya, S. N., Habermacher, R., Martine, U., Closs, E. I. & Filipowicz, W. Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell 125, 1111–1124 (2006).
Miles, W. O., Tschöp, K., Herr, A., Ji, J. Y. & Dyson, N. J. Pumilio facilitates miRNA regulation of the E2F3 oncogene. Genes Dev. 26, 356–368 (2012).
Ke, S. et al. A majority of m6A residues are in the last exons, allowing the potential for 3′ UTR regulation. Genes Dev. 29, 2037–2053 (2015).
Huppertz, I. et al. iCLIP: protein-RNA interactions at nucleotide resolution. Methods 65, 274–287 (2014).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17, 10–12 (2011).
Li, H. & Durbin, R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 26, 589–595 (2010).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 (2015).
Lianoglou, S., Garg, V., Yang, J. L., Leslie, C. S. & Mayr, C. Ubiquitously transcribed genes use alternative polyadenylation to achieve tissue-specific expression. Genes Dev. 27, 2380–2396 (2013).
Acknowledgements
We thank A. Chaudhry and other laboratory members for experimental assistance and discussions. This work was supported by National Institutes of Health grants Al034206 (A.Y.R.), HG007893 (A.Y.R. and C.S.L.), CA164190 (C.S.L.), and P30 CA008748, as well as the Hilton-Ludwig Cancer Prevention Initiative funded by the Conrad N. Hilton Foundation and Ludwig Cancer Research. J.-P. H. was supported by an Irvington Fellowship of the Cancer Research Institute. A.Y.R. is supported as an Investigator with the Howard Hughes Medical Institute. We thank J. Chaudhuri (Memorial Sloan Kettering Cancer Center) for providing antibodies.
Author information
Authors and Affiliations
Contributions
J.-P.H., G.B.L., and A.Y.R. designed the study. J.-P.H. carried out all experiments. Y.L. and C.S.L. designed analytical tools and performed data analyses. J.-P.H., Y.L., and A.Y.R. wrote the manuscript; all authors edited and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
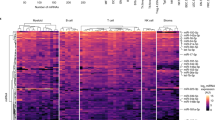
Supplementary Figure 1 Overview of the iCLIP libraries.
(a) miR-155 expression was induced upon activation in B cells, dendritic cells, macrophages, and CD4+ T cells. Error bars represent the standard error estimated from three independent samples (n = 3). (b) Densogram showed RNA-Ago2 complex under digestion of RNase I of different concentrations. UV cross-linked RNA-Ago2 was immunoprecipitated from dendritic cells, and RNAs were labeled with P32isotope at 5’ ends. (c) Macrophage microRNA expression levels in wild type and miR-155 KO cells as measured by iCLIP. Top 25 microRNAs with highest expression in wild type were highlighted; miR-155-5p and miR-139-3p, the most down-regulated and most up-regulated miRNAs in miR-155 KO cells, were labeled. (d) Reproducibility between iCLIP libraries of the same cell type and genotype. Pearson correlation coefficients for the log-transformed iCLIP read counts (log2(read count + 1)) within peaks were computed. In each pairwise comparison, peaks with < 5 reads are excluded. (e) The proportions of iCLIP reads mapped to different genomic categories in each library. Analyses of data from four independent iCLIP experiments are shown
Supplementary Figure 2 Expression and regulation of miR-155-target mRNAs.
(a) Pairwise comparison of WT mRNA expression levels for co-expressed genes (wild-type FPKM > 1 in both cells and difference < 16-fold) containing miR-155 dependent sites. In each comparison, the distributions of RNA-Seq FPKM values for common and cell-type specific target genes were shown separately. (b) Correlation between the FDR of miR-155 dependent iCLIP sites and the regulation of corresponding mRNAs in four cell types. Spearman correlation coefficients and P-values were computed separately for CDS (grey) and 3’UTR (black) sites. Data are representative of three independent RNA-Seq experiments
Supplementary Figure 3 Noncanonical miR-155-target sites and regulation.
(a) Number of miR-155 target sites per cell, including non-canonical sites. (b) Seed type composition of miR-155 dependent sites in co-expressed genes, including non-canonical sites. (c) Averaged profiles of normalized iCLIP read coverages around both canonical and non-canonical miR-155 sites in both wild-type (solid lines) and miR-155 deficient libraries (dotted lines) for all four cell types. Non-canonical miR-155 sites are represented with lighter colors. (d) Examples of non-canonical miR-155 target sites identified by differential iCLIP in all four cell types and in 3’UTRs containing no canonical miR-155 target sites, showing the miRNA-mRNA duplexes predicted by chimiRic and the mRNA-level regulation (log2 fold-changes from RNA-Seq) of corresponding genes. (e) Regulation of mRNA expression by non-canonical miR-155 sites in 3’UTR. The distributions of expression fold changes were shown for multiple sets of target genes with only non-canonical 3’UTR miR-155 sites selected by different FDR cutoffs, along with the genes containing canonical 3’UTR miR-155 seed matches and the genes containing canonical 3’UTR miR-155 dependent iCLIP sites for comparison. Data are representatives of independent iCLIP (n = 4) and RNA-Seq (n = 3) experiments
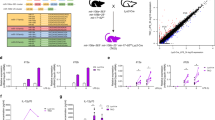
Supplementary Figure 4 Summary of quantification of protein levels of miR-155 targets.
(a) Quantification of protein levels of miR-155 targets. Representative western blot analyses of indicated protein expression in B cells (b), dendritic cells (c), and macrophages (d). Repression was determined based on normalized protein levels in wild type vs. miR-155 KO cells. Error bar displays standard error from at least three biologically independent samples; P-value was measured by two-sided t-test. * P < 0.05, ** P < 0.01
Supplementary Figure 5 Summary of miR-155-dependent sites identified from differential iCLIP.
(a) The number of miR-155 dependent iCLIP sites per gene (including the ones within introns and ncRNAs) in each cell type. (b) Top five genes ranked by the normalized abundance of WT iCLIP reads within miR-155 dependent sites in each cell type. Data are representative of four independent iCLIP experiments
Supplementary Figure 6 Usage of 3′-UTR isoforms across four cell types characterized by poly(A)-seq.
(a) The changes in 3’UTR isoform usage for 3’UTRs with two major isoforms at 24 h after CD4+ T cell activation. Highlighted were 3’UTRs with significant usage changes. Data are representative of three independent PolyA-Seq experiments. (b) Correlation between the abundances of RNA-Seq (FPKM) and PolyA-Seq (FPM) for genes with a single 3’UTR isoform in wild-type cells. Spearman correlation coefficients were also shown. (c) Length distribution of all 3’UTR isoforms characterized by PolyA-Seq. (d) Correlation between miR-155 mediated repression per isoform and distance from miR-155 binding sites to the polyA ends. Spearman correlation coefficient and P-value were displayed for each cell type. Analyses of data from independent RNA-Seq (n = 3) and PolyA-Seq (n = 4) experiments are shown
Supplementary Figure 7 Effect of differential ApA usage on context-specific miR-155 regulation.
(a) The effect of ApA isoform usage on the regulation of miR-155 sites in multi-isoform 3’UTRs. In each individual cell type, the usage of 3’UTR isoforms containing miR-155 site is plotted against the gene-level regulation (RNA-Seq log2(ko/wt)). Spearman correlation coefficient and P-value were displayed for each cell. (b) Venn diagrams show the number of miR-155 target genes affected by differential ApA usage in each pairwise comparison. Analyses of data from independent RNA-Seq (n = 3) and PolyA-Seq (n = 4) experiments are shown
Supplementary Figure 8 Summary of iCLIP peaks.
Proportion of top iCLIP peaks (ranked by normalized read counts in wild-type libraries) containing miR-155 seed matches that correspond to differentially bound miR-155 sites. Data are representative of four independent iCLIP experiments
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8, Supplementary Tables 2, 5, 9 and 10, and Supplementary Note
Supplementary Table 1
Number of unique iCLIP reads mapped to all high-confidence mature mouse miRNAs from miRBase in each iCLIP library
Supplementary Table 3
Summary of all significant (FDR < 2.5%) miR-155 dependent sites in mRNAs across four immune cells. The information includes the genomic coordinates and annotations of miR-155 seed matches, log2 fold changes and P-values fitted by negative binomial GLM, numbers of unique reads within corresponding iCLIP peaks from all libraries, and the type of miR-155 seed matches
Supplementary Table 4
Summary of differential mRNA expression (only considering RNA-Seq alignments in CDS exons) between wild type and miR-155 KO genotypes in all four immune cells. For each gene, the log2 fold changes and P-values fitted by negative binomial GLM are shown, along with the average FPKM levels in wild type and miR-155 KO libraries
Supplementary Table 6
Summary of all significant (FDR < 2.5%) miR-155 dependent sites in introns and non-coding RNAs across four immune cells, in the same format as Supplementary Table 3
Supplementary Table 7
Summary of ApA usage at three time points during CD4+ T cell activation (0h, 24h and 48h after activation) captured by PolyA-Seq. The information includes the genomic coordinates and annotations of PolyA-Seq peaks, along with numbers of reads within peaks from all libraries
Supplementary Table 8
Summary of ApA usage at 48 h after immune activation across four immune cells captured by PolyA-Seq, in the same format as Supplementary Table 7
Rights and permissions
About this article
Cite this article
Hsin, JP., Lu, Y., Loeb, G.B. et al. The effect of cellular context on miR-155-mediated gene regulation in four major immune cell types. Nat Immunol 19, 1137–1145 (2018). https://doi.org/10.1038/s41590-018-0208-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-018-0208-x
This article is cited by
-
MicroRNA as a potential biomarker for systemic lupus erythematosus: pathogenesis and targeted therapy
Clinical and Experimental Medicine (2023)
-
Development trends of immune activation during HIV infection in recent three decades: a bibliometric analysis based on CiteSpace
Archives of Microbiology (2023)
-
The deleted in oral cancer (DOC1 aka CDK2AP1) tumor suppressor gene is downregulated in oral squamous cell carcinoma by multiple microRNAs
Cell Death & Disease (2023)
-
Systemic Listeria monocytogenes infection in aged mice induces long-term neuroinflammation: the role of miR-155
Immunity & Ageing (2022)
-
MiR155 Disrupts the Intestinal Barrier by Inducing Intestinal Inflammation and Altering the Intestinal Microecology in Severe Acute Pancreatitis
Digestive Diseases and Sciences (2022)