Abstract
N-myristoyltransferase (NMT) attaches the fatty acid myristate to the N-terminal glycine of proteins to sort them into soluble and membrane-bound fractions. Function of the energy-sensing AMP-activated protein kinase, AMPK, is myristoylation dependent. In rheumatoid arthritis (RA), pathogenic T cells shift glucose away from adenosine tri-phosphate production toward synthetic and proliferative programs, promoting proliferation, cytokine production, and tissue invasion. We found that RA T cells had a defect in NMT1 function, which prevented AMPK activation and enabled unopposed mTORC1 signaling. Lack of the myristate lipid tail disrupted the lysosomal translocation and activation of AMPK. Instead, myristoylation-incompetent RA T cells hyperactivated the mTORC1 pathway and differentiated into pro-inflammatory TH1 and TH17 helper T cells. In vivo, NMT1 loss caused robust synovial tissue inflammation, whereas forced NMT1 overexpression rescued AMPK activation and suppressed synovitis. Thus, NMT1 has tissue-protective functions by facilitating lysosomal recruitment of AMPK and dampening mTORC1 signaling.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
References
Rao, D. A. et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature 542, 110–114 (2017).
Weyand, C. M. & Goronzy, J. J. T-cell-targeted therapies in rheumatoid arthritis. Nat. Clin. Pract. Rheumatol. 2, 201–210 (2006).
Weyand, C. M. & Goronzy, J. J. Immunometabolism in early and late stages of rheumatoid arthritis. Nat. Rev. Rheumatol. 13, 291–301 (2017).
Weyand, C. M., Shen, Y. & Goronzy, J. Redox-sensitive signaling in inflammatory T cells and in autoimmune disease. Free Radic. Biol. Med. 125, 36–43 (2018).
Shen, Y. et al. Metabolic control of the scaffold protein TKS5 in tissue-invasive, proinflammatory T cells. Nat. Immunol. 18, 1025–1034 (2017).
Yang, Z., Fujii, H., Mohan, S. V., Goronzy, J. J. & Weyand, C. M. Phosphofructokinase deficiency impairs ATP generation, autophagy, and redox balance in rheumatoid arthritis T cells. J. Exp. Med. 210, 2119–2134 (2013).
Yang, Z. et al. Restoring oxidant signaling suppresses proarthritogenic T cell effector functions in rheumatoid arthritis. Sci. Transl. Med. 8, 331ra338 (2016).
Tsokos, G. C. Metabolic control of arthritis: switch pathways to treat. Sci. Transl. Med. 8, 331fs338 (2016).
Shao, L. et al. Deficiency of the DNA repair enzyme ATM in rheumatoid arthritis. J. Exp. Med. 206, 1435–1449 (2009).
Li, Y. et al. Deficient activity of the nuclease MRE11A induces T cell aging and promotes arthritogenic effector functions in patients with rheumatoid arthritis. Immunity 45, 903–916 (2016).
Tsokos, G. C. Fat T cells go to the joint. Nat. Immunol. 18, 955–956 (2017).
Wang, C. W. Lipid droplets, lipophagy, and beyond. Biochim. Biophys. Acta 1861, 793–805 (2016).
Ma, E. H., Poffenberger, M. C., Wong, A. H. & Jones, R. G. The role of AMPK in T cell metabolism and function. Curr. Opin. Immunol. 46, 45–52 (2017).
Gowans, G. J., Hawley, S. A., Ross, F. A. & Hardie, D. G. AMP is a true physiological regulator of AMP-activated protein kinase by both allosteric activation and enhancing net phosphorylation. Cell. Metab. 18, 556–566 (2013).
Herzig, S. & Shaw, R. J. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 19, 121–135 (2018).
Zhang, C. S. et al. The lysosomal v-ATPase-Ragulator complex is a common activator for AMPK and mTORC1, acting as a switch between catabolism and anabolism. Cell. Metab. 20, 526–540 (2014).
Lin, S. C. & Hardie, D. G. AMPK: sensing glucose as well as cellular energy status. Cell. Metab. 27, 299–313 (2018).
Zhang, C. S. et al. Fructose-1,6-bisphosphate and aldolase mediate glucose sensing by AMPK. Nature 548, 112–116 (2017).
Blagih, J. et al. The energy sensor AMPK regulates T cell metabolic adaptation and effector responses in vivo. Immunity 42, 41–54 (2015).
Kim, J., Kundu, M., Viollet, B. & Guan, K. L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 13, 132–141 (2011).
Chi, H. Regulation and function of mTOR signalling in T cell fate decisions. Nat. Rev. Immunol. 12, 325–338 (2012).
Delgoffe, G. M. et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat. Immunol. 12, 295–303 (2011).
Sancak, Y. et al. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 141, 290–303 (2010).
Hardie, D. G. AMPK and Raptor: matching cell growth to energy supply. Mol. Cell 30, 263–265 (2008).
Oakhill, J. S. et al. beta-Subunit myristoylation is the gatekeeper for initiating metabolic stress sensing by AMP-activated protein kinase (AMPK). Proc. Natl Acad. Sci. USA 107, 19237–19241 (2010).
Udenwobele, D. I. et al. Myristoylation: an important protein modification in the immune response. Front. Immunol. 8, 751 (2017).
Ducker, C. E., Upson, J. J., French, K. J. & Smith, C. D. Two N-myristoyltransferase isozymes play unique roles in protein myristoylation, proliferation, and apoptosis. Mol. Cancer Res. 3, 463–476 (2005).
Yang, S. H. et al. N-myristoyltransferase 1 is essential in early mouse development. J. Biol. Chem. 280, 18990–18995 (2005).
Shrivastav, A. et al. Requirement of N-myristoyltransferase 1 in the development of monocytic lineage. J. Immunol. 180, 1019–1028 (2008).
Weyand, C. M., Yang, Z. & Goronzy, J. J. T-cell aging in rheumatoid arthritis. Curr. Opin. Rheumatol. 26, 93–100 (2014).
Finlay, D. & Cantrell, D. A. Metabolism, migration and memory in cytotoxic T cells. Nat. Rev. Immunol. 11, 109–117 (2011).
Navarro, M. N. & Cantrell, D. A. Serine-threonine kinases in TCR signaling. Nat. Immunol. 15, 808–814 (2014).
Gwinn, D. M. et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell 30, 214–226 (2008).
Zhang, C. S. et al. Metformin activates AMPK through the lysosomal pathway. Cell. Metab. 24, 521–522 (2016).
Fullerton, M. D. et al. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat. Med. 19, 1649–1654 (2013).
Lochner, M., Berod, L. & Sparwasser, T. Fatty acid metabolism in the regulation of T cell function. Trends Immunol. 36, 81–91 (2015).
Dudek, E. et al. N-Myristoyltransferase 1 interacts with calnexin at the endoplasmic reticulum. Biochem. Biophys. Res. Commun. 468, 889–893 (2015).
Ohta, H., Takamune, N., Kishimoto, N., Shoji, S. & Misumi, S. N-Myristoyltransferase 1 enhances human immunodeficiency virus replication through regulation of viral RNA expression level. Biochem. Biophys. Res. Commun. 463, 988–993 (2015).
Kim, S. et al. Blocking myristoylation of SRC inhibits its kinase activity and suppresses prostate cancer progression. Cancer Res. 77, 6950–6962 (2017).
Thinon, E. et al. Global profiling of co- and post-translationally N-myristoylated proteomes in human cells. Nat. Commun. 5, 4919 (2014).
Liang, J. et al. Myristoylation confers noncanonical AMPK functions in autophagy selectivity and mitochondrial surveillance. Nat. Commun. 6, 7926 (2015).
Hardie, D. G. AMPK—sensing energy while talking to other signaling pathways. Cell. Metab. 20, 939–952 (2014).
Kelly, B., Tannahill, G. M., Murphy, M. P. & O’Neill, L. A. Metformin inhibits the production of reactive oxygen species from NADH:ubiquinone oxidoreductase to limit induction of interleukin-1beta (IL-1beta) and boosts interleukin-10 (IL-10) in lipopolysaccharide (LPS)-activated macrophages. J. Biol. Chem. 290, 20348–20359 (2015).
O’Neill, L. A., Kishton, R. J. & Rathmell, J. A guide to immunometabolism for immunologists. Nat. Rev. Immunol. 16, 553–565 (2016).
Perl, A. Review: metabolic control of immune system activation in rheumatic diseases. Arthritis Rheumatol. 69, 2259–2270 (2017).
Cool, B. et al. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell. Metab. 3, 403–416 (2006).
Lai, Z. W. et al. Sirolimus in patients with clinically active systemic lupus erythematosus resistant to, or intolerant of, conventional medications: a single-arm, open-label, phase 1/2 trial. Lancet 391, 1186–1196 (2018).
Huang, N. & Perl, A. Metabolism as a target for modulation in autoimmune diseases. Trends Immunol. 39, 562–576 (2018).
Wen, Z. et al.The microvascular niche instructs T cells in large vessel vasculitis via the VEGF-Jagged1-Notch pathway. Sci. Transl. Med. 9, pii: eaal3322 (2017).
Fang, Y. et al. Duration of rapamycin treatment has differential effects on metabolism in mice. Cell. Metab. 17, 456–462 (2013).
Acknowledgements
This work was supported by the National Institutes of Health (grant nos. R01 AR042527, R01 HL 117913, R01 AI108906 and P01 HL129941 to C.M.W. and nos. R01 AI108891, R01 AG045779, U19 AI057266 and I01 BX001669 to J.J.G.).
Author information
Authors and Affiliations
Contributions
Z.W., C.M.W., and J.J.G. designed the study and analyzed the data. Z.W., K.J., Y.S., Z.Y., Y.L., and B.W. performed experiments. S.S., N.E.R., C.M.W., and J.J.G. were responsible for patient selection, evaluation, and recruitment. L.T. supervised all statistical analyses. C.M.W., Z.W., and J.J.G. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
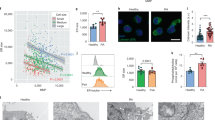
Supplementary Figure 1 Expression of NMT1 and NMT2.
(a) CD4+CD45RA+ T cells were isolated from patients with rheumatoid arthritis (RA), patients with psoriatic arthritis (PsA) or age-matched healthy individuals and stimulated for 72 h. NMT2 expression was analyzed by flow cytometry. Data (mean ± SEM) from 10 RA patients, 8 PsA patients and 10 healthy individuals. One-way ANOVA and post-ANOVA pair-wise two-group comparisons conducted with Tukey’s method. (b) CD4+CD45RA+ T cells were stimulated for 72 h and NMT1 mRNA expression was measured by RT-PCR. Mean ± SEM from 10 samples in each group. Unpaired Mann-Whitney-Wilcoxon rank test. (c-h) PBMCs were collected from RA patients, PsA patients and healthy individuals. Multicolor flow cytometry was applied to quantify NMT1 expression in CD4+CD45RA+ T cells, CD4+CD45RA– T cells, CD8+CD45RA+ T cells, CD8+CD45RA– T cells, CD19+ B cells and CD14+ monocytes. Representative histograms and collective MFIs (mean ± SEM) for each of the subpopulations from 11 individuals in each donor cohort. One-way ANOVA and post-ANOVA pair-wise two-group comparisons conducted with Tukey’s method. **p < 0.01. ***p < 0.001
Supplementary Figure 2 Genetic manipulation of NMT1 expression in CD4+ T cells.
(a) CD4+CD45RA+ T cells from RA patients were transfected with a NMT1 expression vector or a control vector. (b) CD4+CD45RA+ T cells from healthy subjects were transfected with NMT1 siRNA or control siRNA. 24 h later, NMT1 protein expression was analyzed with flow cytometry. Representative histograms and collective data from 6 individuals in each group. Paired Mann-Whitney-Wilcoxon rank test. *p < 0.05
Supplementary Figure 3 NMT1 regulates lipid droplet accumulation in T cells.
CD4+CD45RA+ T cells from RA patients and healthy donors were stimulated for 72 h. Neutral lipid droplets were stained with Bodipy 493/503 and analyzed by flow cytometry. (a) Comparison of neutral lipid expression in control and RA T cells. Representative histograms and results from 6 patient-control pairs. Mean ± SEM. **p < 0.01 by paired Mann-Whitney-Wilcoxon rank test. (b) NMT1 activity was restored in T cells from RA patients by transfecting a NMT1-expressing vector. Lipid droplets were detected by staining with Bodipy. Representative histograms are shown. (c) NMT1 activity was inhibited by transfecting T cells from healthy individuals with NMT1 siRNA. Histograms of Bodipy staining for lipid droplets are shown.
Supplementary Figure 4 NMT1 and NMT2 expression in CD4+ T cells is independent of metabolic signals.
Naïve CD4 T cells from 6 healthy donors were stimulated for 72 h in the absence or presence of C75 (20 μM), 3PO (200 nM), ML265 (10 μM), Shikonin (250 nM), pyruvate (1 mM), succinate (1 mM), malic acid (1 mM), A769662 (10 μM), Compound C (1 μM) or rapamycin (10 μM), respectively. Protein expression of NMT1 and NMT2 in CD4 T cells was determined by flow cytometry. One-way ANOVA and post-ANOVA pair-wise two-group comparisons were conducted with Tukey’s method
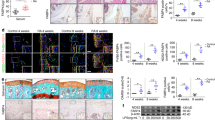
Supplementary Figure 5 N-myristoylation protects against synovial inflammation.
(a) Scheme of experimental design. NSG mice were engrafted with human synovial tissue. Seven days later, CD4 T cells were FACS sorted from CD4+CD45RO– PBMCs of RA patients or healthy individuals, manipulated for NMT1 expression by transfecting with a NMT1 vector, NMT1 siRNA or controls respectively, and added back to the CD4-depleted PBMCs for adoptive transfer into the chimeras. In other experiments, CD45RO– PBMCs were transfected. At day 14, synovial tissues were harvested for transcriptome analysis and immunohistochemical staining. (b-e) NMT1 overexpression inhibits synovial inflammation. NSG mice were engrafted with human synovial tissue. Seven days after engraftment, CD45RO– PBMCs from RA patients were transfected with a NMT1-expressing or control vector and transferred into the chimeric mice. Seven days later, synovial tissues were harvested for transcriptome analysis and immunostaining. (b) Tissue sections stained with anti-IFN-γ (green) and anti-CD3 (red). Nuclei marked with DAPI. Representative images from 6 grafts. Scale bars 20 μm. (c–d) Frequencies of CD3+ T cells and of CD3+IFN-γ+ T cells in tissue sections. (e) Heat map presentation of gene transcripts measured in tissue extracts by qPCR. Data are mean ± SEM from 6 synovial grafts. *p < 0.05 by paired Mann-Whitney-Wilcoxon rank test. (f-i) NMT1 knockdown promotes synovial inflammation. Human synovial tissue was transplanted into NSG mice. After engraftment, CD45RO– PBMCs from healthy donors were transfected with NMT1 siRNA or control siRNA and adoptively transferred into the chimeric mice. Tissue transcriptome and immunohistochemical stains were analyzed in synovial explants after 7 days. (f) Tissue-infiltrating human T cells evaluated by dual-color immunostaining of CD3 (red) and IFN-γ (green). Nuclei marked with DAPI. Representative images from 6 grafts. Scale bars 20 μm. (g-h) Frequencies of tissue CD3+ T cells and of CD3+IFN-γ+ T cells. (i) Tissue transcriptome of inflammation-associated genes shown as a heat map. Data are mean ± SEM from 6 synovial grafts. *p < 0.05 by paired Mann-Whitney-Wilcoxon rank test
Supplementary Figure 6 AMPK activity and transcriptional expression in healthy individuals and RA patients.
CD4+CD45RA+ T cells were purified from RA patients or healthy individuals and stimulated for 72 h. (a) AMPK activity was determined by analyzing inhibitory phosphorylation of acetyl-CoA carboxylase (ACC). Protein expression of phospho-ACC was determined by Western blotting. β-actin served as loading control. Representative immunoblots and relative intensities (mean ± SEM) from 5 RA-healthy pairs. Each dot represents the data from one donor. *p < 0.05 by unpaired Mann-Whitney-Wilcoxon rank test. (b) Transcripts for AMPK complex components were analyzed by qPCR. Data are mean ± SEM from 7 RA-healthy pairs.
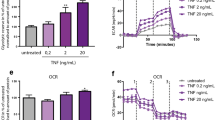
Supplementary Figure 7 AMPK regulates mTORC1 activation but not its lysosomal localization.
(a) Naïve CD4+ T cells were isolated from healthy individuals or RA patients and stimulated for 72 h. Lysosomes were identified with antibodies to human LAMP1 (red). mTOR was stained with anti-mTOR (green). LAMP1-mTOR co-localization was analyzed by confocal microscopy. Each dot represents one T cell. Scale bars 20 μm. Unpaired Mann-Whitney-Wilcoxon rank test. (b, c) Naïve CD4+ T cells from healthy donors were transfected with AMPKa siRNA or control siRNA and stimulated for 72 h. (b) AMPKα protein expression was analyzed by flow cytometry after 24 hrs. ***p < 0.001 by paired student’s t-test. (c) mTOR expression was quantified in lysosomal fractions and in whole cell lysates. Representative immunoblots from 4 individuals in each group. (d) AMPK-dependent inhibition of mTORC1 activity in T cells. Naïve CD4+ T cells from 6 healthy individuals were stimulated for 72 h in the absence or presence of the AMPK inhibitor Compound C (1 μM). Phospho-S6RP in CD4+ T cells was determined by phosflow cytometry. Paired Mann-Whitney-Wilcoxon rank test. *p < 0.05
Supplementary Figure 8 Anti-inflammatory effects of AMPK activation and mTORC1 inhibition.
(a) The AMPK activator A769662 does not affect Th2 and Treg lineage markers in vivo. NSG mice were implanted with human synovial tissues, reconstituted with CD45RO– PBMCs from RA patients, and randomly assigned to control arm (vehicle) or treatment arm (A769662, 30 mg/kg/mouse, twice a day). Synovial grafts were explanted and analyzed for mRNA expression of Th2 and Treg lineage markers (GATA3, IL4, FOXP3) after 7 days of treatment. Data are mean ± SEM from 6 grafts in each group (paired Mann-Whitney-Wilcoxon rank test). (b) The mTORC1 inhibitor rapamycin does not affect Th2 and Treg lineage markers in vivo. NSG mice were implanted with human synovial tissues, reconstituted with CD45RO– PBMCs from RA patients, and randomly assigned to control arm (vehicle) and treatment arm (rapamycin, 5 mg/kg/mouse, every other day). Synovial grafts were explanted and analyzed for mRNA expression of Th2 and Treg lineage markers (GATA3, IL4, FOXP3) after 9 days of treatment. Data are mean ± SEM from 6 grafts in each group. Paired Mann-Whitney-Wilcoxon rank test. (c) mTORC1 inhibition abrogates differentiation of RA T cells into pro-inflammatory effector cells. Naïve CD4+ T cells from 6 RA patients were stimulated in the absence or presence of the mTORC1 inhibitor rapamycin (Rapa, 10 μM). Lineage-determining transcription factors and signature cytokines were analyzed. Each dot represents the data from one patient. Paired Mann-Whitney-Wilcoxon rank test. *p < 0.05. (d) Effect of A769662 on CD4+ Treg cell induction in vitro. CD4+CD45RA+ T cells from RA patients were stimulated in the presence of an increasing dose of the AMPK activator A769662 for 4 days and expression of the Treg lineage-determining transcription factor FoxP3 was analyzed by flow cytometry. Data (mean ± SEM) from 6 RA patients. One-way ANOVA and post-ANOVA pair-wise two-group comparisons were conducted with Tukey’s method. ***p < 0.001
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8, Supplementary Table 1
Rights and permissions
About this article
Cite this article
Wen, Z., Jin, K., Shen, Y. et al. N-myristoyltransferase deficiency impairs activation of kinase AMPK and promotes synovial tissue inflammation. Nat Immunol 20, 313–325 (2019). https://doi.org/10.1038/s41590-018-0296-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-018-0296-7
This article is cited by
-
Protein lipidation in health and disease: molecular basis, physiological function and pathological implication
Signal Transduction and Targeted Therapy (2024)
-
Cancer CD39 drives metabolic adaption and mal-differentiation of CD4+ T cells in patients with non-small-cell lung cancer
Cell Death & Disease (2023)
-
Myristic acid as a checkpoint to regulate STING-dependent autophagy and interferon responses by promoting N-myristoylation
Nature Communications (2023)
-
Aging-associated HELIOS deficiency in naive CD4+ T cells alters chromatin remodeling and promotes effector cell responses
Nature Immunology (2023)
-
Metformin combined with rapamycin ameliorates podocyte injury in idiopathic membranous nephropathy through the AMPK/mTOR signaling pathway
Journal of Cell Communication and Signaling (2023)