Abstract
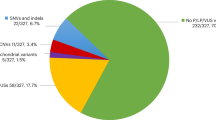
Cerebral palsy (CP) is the most common motor disability in children. To ascertain the role of major genetic variants in the etiology of CP, we conducted exome sequencing on a large-scale cohort with clinical manifestations of CP. The study cohort comprised 505 girls and 1,073 boys. Utilizing the current gold standard in genetic diagnostics, 387 of these 1,578 children (24.5%) received genetic diagnoses. We identified 412 pathogenic and likely pathogenic (P/LP) variants across 219 genes associated with neurodevelopmental disorders, and 59 P/LP copy number variants. The genetic diagnostic rate of children with CP labeled at birth with perinatal asphyxia was higher than the rate in children without asphyxia (P = 0.0033). Also, 33 children with CP manifestations (8.5%, 33 of 387) had findings that were clinically actionable. These results highlight the need for early genetic testing in children with CP, especially those with risk factors like perinatal asphyxia, to enable evidence-based medical decision-making.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The sequencing data from our patients have been deposited securely in the Genome Sequence Archive (GSA) housed within the National Genomics Data Center at the China National Center for Bioinformation/Beijing Institute of Genomics, Chinese Academy of Sciences. The raw sequencing data are available under restricted access in GSA-Human (BioProject accession no.: PRJCA023830 (https://ngdc.cncb.ac.cn/gsa-human/)) due to patient privacy and Regulations on the Management of Human Genetics Resources of China, following the GSA guidelines (https://ngdc.cncb.ac.cn/gsa-human/document). VCF files of 1,578 children with clinical manifestations of CP have been deposited in the National Human Genetic Resources system (Record no.: *BF2023081113944). For access to data, please email qhxing@fudan.edu.cn or changlian.zhu@neuro.gu.se. The committee reviews data access requests on a monthly basis. This statement pertains to the distribution of sequencing data produced by our research. In addition, the P/LP/VUS variants within our CP cohort and previously reported P/LP/VUS variants in other CP cohorts are accessible at http://81.70.179.157/ all the time. The databases used in our analysis are all publicly available and can be obtained from the following links: ClinVar: https://www.ncbi.nlm.nih.gov/clinvar; Gene Ontology: http://geneontology.org; gnomAD: http://www.gnomad-sg.org; and Kyoto Encyclopedia of Genes and Genomes (KEGG): https://www.genome.jp/kegg.
Code availability
All codes used in this manuscript have been deposited and are publicly available at https://github.com/YeCheng57/CerebralPalsy_pipeline.
References
Yeargin-Allsopp, M. et al. Prevalence of cerebral palsy in 8-year-old children in three areas of the United States in 2002: a multisite collaboration. Pediatrics 121, 547–554 (2008).
Lee, R. W. et al. A diagnostic approach for cerebral palsy in the genomic era. Neuromolecular Med. 16, 821–844 (2014).
Beysen, D. et al. Genetic testing contributes to diagnosis in cerebral palsy: Aicardi-Goutieres syndrome as an example. Front Neurol. 12, 617813 (2021).
Chang, M. J., Ma, H. I. & Lu, T. H. Estimating the prevalence of cerebral palsy in Taiwan: a comparison of different case definitions. Res. Dev. Disabil. 36C, 207–212 (2015).
MacLennan, A. H., Thompson, S. C. & Gecz, J. Cerebral palsy: causes, pathways, and the role of genetic variants. Am. J. Obstet. Gynecol. 213, 779–788 (2015).
Smithers-Sheedy, H. et al. A special supplement: findings from the Australian Cerebral Palsy Register, birth years 1993 to 2006. Dev. Med. Child Neurol. 58, 5–10 (2016).
Sellier, E. et al. Trends in prevalence of cerebral palsy in children born with a birthweight of 2,500 g or over in Europe from 1980 to 1998. Eur. J. Epidemiol. 25, 635–642 (2010).
Gonzalez-Mantilla, P. J. et al. Diagnostic yield of exome sequencing in cerebral palsy and implications for genetic testing guidelines: a systematic review and meta-analysis. JAMA Pediatr. 177, 472–478 (2023).
Moreno-De-Luca, A., Ledbetter, D. H. & Martin, C. L. Genetic insights into the causes and classification of cerebral palsies. Lancet Neurol. 11, 283–292 (2012).
Hemminki, K., Li, X. J., Sundquist, K. & Sundquist, J. High familial risks for cerebral palsy implicate partial heritable aetiology. Paediatr. Perinat. Epidemiol. 21, 235–241 (2007).
Tollanes, M. C., Wilcox, A. J., Lie, R. T. & Moster, D. Familial risk of cerebral palsy: population based cohort study. BMJ 349, g4294 (2014).
Zhang, G. et al. Genetic associations with gestational duration and spontaneous preterm birth. N. Engl. J. Med. 377, 1156–1167 (2017).
Lynex, C. N. et al. Homozygosity for a missense mutation in the 67 kDa isoform of glutamate decarboxylase in a family with autosomal recessive spastic cerebral palsy: parallels with Stiff-Person Syndrome and other movement disorders. BMC Neurol. 4, 20 (2004).
Lerer, I. et al. Deletion of the ANKRD15 gene at 9p24.3 causes parent-of-origin-dependent inheritance of familial cerebral palsy. Hum. Mol. Genet. 14, 3911–3920 (2005).
Abou Jamra, R. et al. Adaptor protein complex 4 deficiency causes severe autosomal-recessive intellectual disability, progressive spastic paraplegia, shy character, and short stature. Am. J. Hum. Genet. 88, 788–795 (2011).
Moreno-De-Luca, A. et al. Adaptor protein complex-4 (AP-4) deficiency causes a novel autosomal recessive cerebral palsy syndrome with microcephaly and intellectual disability. J. Med. Genet. 48, 141–144 (2011).
Verkerk, A. J. et al. Mutation in the AP4M1 gene provides a model for neuroaxonal injury in cerebral palsy. Am. J. Hum. Genet. 85, 40–52 (2009).
Kruer, M. C. et al. Mutations in gamma adducin are associated with inherited cerebral palsy. Ann. Neurol. 74, 805–814 (2013).
Hirata, H. et al. ZC4H2 mutations are associated with arthrogryposis multiplex congenita and intellectual disability through impairment of central and peripheral synaptic plasticity. Am. J. Hum. Genet. 92, 681–695 (2013).
van Eyk, C., MacLennan, S. C. & MacLennan, A. H. All patients with a cerebral palsy diagnosis merit genomic sequencing. JAMA Pediatr. 177, 455–456 (2023).
Liu, J. C. et al. Multi-omics research in sarcopenia: current progress and future prospects. Ageing Res. Rev. 76, 101576 (2022).
Srivastava, S. et al. Molecular diagnostic yield of exome sequencing and chromosomal microarray in cerebral palsy: a systematic review and meta-analysis. JAMA Neurol. 79, 1287–1295 (2022).
Nagy, E. et al. The usefulness of MRI Classification System (MRICS) in a cerebral palsy cohort. Acta Paediatr. 109, 2783–2788 (2020).
Himmelmann, K. et al. MRI classification system (MRICS) for children with cerebral palsy: development, reliability, and recommendations. Dev. Med. Child Neurol. 59, 57–64 (2017).
Paneth, N. & Stark, R. I. Cerebral palsy and mental retardation in relation to indicators of perinatal asphyxia. An epidemiologic overview. Am. J. Obstet. Gynecol. 147, 960–966 (1983).
van Rappard, D. F., Boelens, J. J. & Wolf, N. I. Metachromatic leukodystrophy: disease spectrum and approaches for treatment. Best. Pract. Res. Clin. Endocrinol. Metab. 29, 261–273 (2015).
Lerner-Ellis, J. P. et al. Identification of the gene responsible for methylmalonic aciduria and homocystinuria, cblC type. Nat. Genet. 38, 93–100 (2006).
Allewelt, H. et al. Long-term functional outcomes after hematopoietic stem cell transplant for early infantile Krabbe disease. Biol. Blood Marrow Transpl. 24, 2233–2238 (2018).
Zhong, C. et al. M6A-modified circRBM33 promotes prostate cancer progression via PDHA1-mediated mitochondrial respiration regulation and presents a potential target for ARSI therapy. Int J. Biol. Sci. 19, 1543–1563 (2023).
Haussler, M., Hoffmann, G. F. & Wevers, R. A. l-DOPA and selegiline for tyrosine hydroxylase deficiency. J. Pediatr. 138, 451–452 (2001).
Huppke, P. et al. Activating de novo mutations in NFE2L2 encoding NRF2 cause a multisystem disorder. Nat. Commun. 8, 818 (2017).
Friedman, J., Hyland, K., Blau, N. & MacCollin, M. DOPA-responsive hypersomnia and mixed movement disorder due to sepiapterin reductase deficiency. Neurology 67, 2032–2035 (2006).
Moreno-De-Luca, A. et al. Molecular diagnostic yield of exome sequencing in patients with cerebral palsy. JAMA 325, 467–475 (2021).
Ioannidis, N. M. et al. REVEL: an ensemble method for predicting the pathogenicity of rare missense variants. Am. J. Hum. Genet. 99, 877–885 (2016).
Ke, H. N. et al. Landscape of pathogenic mutations in premature ovarian insufficiency. Nat. Med. 29, 483–492 (2023).
McMichael, G. et al. Whole-exome sequencing points to considerable genetic heterogeneity of cerebral palsy. Mol. Psychiatry 20, 176–182 (2015).
Takezawa, Y. et al. Genomic analysis identifies masqueraders of full-term cerebral palsy. Ann. Clin. Transl. Neurol. 5, 538–551 (2018).
Matthews, A. M. et al. Atypical cerebral palsy: genomics analysis enables precision medicine. Genet. Med. 21, 1621–1628 (2019).
Bamshad, M. J., Nickerson, D. A. & Chong, J. X. Mendelian gene discovery: fast and furious with no end in sight. Am. J. Hum. Genet. 105, 448–455 (2019).
van Eyk, C. L. et al. Analysis of 182 cerebral palsy transcriptomes points to dysregulation of trophic signalling pathways and overlap with autism. Transl. Psychiatry 8, 88 (2018).
van Eyk, C. L. et al. Yield of clinically reportable genetic variants in unselected cerebral palsy by whole genome sequencing. NPJ Genom. Med. 6, 74 (2021).
MacLennan, A. A template for defining a causal relation between acute intrapartum events and cerebral palsy: international consensus statement. BMJ 319, 1054–1059 (1999).
O’Brien, J. R., Usher, R. H. & Maughan, G. B. Causes of birth asphyxia and trauma. Can. Med. Assoc. J. 94, 1077–1085 (1966).
Windle, W. F. Neurological and psychological deficits from asphyxia neonatorum. Public Health Rep. (1896) 72, 646–650 (1957).
Phelan, J. P., Martin, G. I. & Korst, L. M. Birth asphyxia and cerebral palsy. Clin. Perinatol. 32, 61–76 (2005).
Sartwelle, T. P., Johnston, J. C. & Arda, B. A half century of electronic fetal monitoring and bioethics: silence speaks louder than words. Matern. Health Neonatol. Perinatol. 3, 21 (2017).
Zarrei, M. et al. De novo and rare inherited copy-number variations in the hemiplegic form of cerebral palsy. Genet. Med. 20, 172–180 (2018).
Han, V. X., Patel, S., Jones, H. F. & Dale, R. C. Maternal immune activation and neuroinflammation in human neurodevelopmental disorders. Nat. Rev. Neurol. 17, 564–579 (2021).
Jung, E. et al. Clinical chorioamnionitis at term: definition, pathogenesis, microbiology, diagnosis, and treatment. Am. J. Obstet. Gynecol. 230, S807–S840 (2024).
Prieto-Villalobos, J. et al. Astroglial hemichannels and pannexons: the hidden link between maternal inflammation and neurological disorders. Int. J. Mol. Sci. 22, 9503 (2021).
Korzeniewski, S. J., Slaughter, J., Lenski, M., Haak, P. & Paneth, N. The complex aetiology of cerebral palsy. Nat. Rev. Neurol. 14, 528–543 (2018).
Smith, L. D., Willig, L. K. & Kingsmore, S. F. Whole-exome sequencing and whole-genome sequencing in critically ill neonates suspected to have single-gene disorders. Cold Spring Harb. Perspect. Med. 6, a023168 (2015).
Smithers-Sheedy, H. et al. What constitutes cerebral palsy in the twenty-first century? Dev. Med. Child Neurol. 56, 323–328 (2014).
Mei, H. et al. Genetic spectrum identified by exome sequencing in a Chinese pediatric cerebral palsy cohort. J. Pediatr. 242, 206–212.e6 (2022).
Chopra, M. et al. Mendelian etiologies identified with whole exome sequencing in cerebral palsy. Ann. Clin. Transl. Neurol. 9, 193–205 (2022).
Oskoui, M. et al. Clinically relevant copy number variations detected in cerebral palsy. Nat. Commun. 6, 7949 (2015).
Chen, A., Dyck Holzinger, S., Oskoui, M., Shevell, M.& Canadian Cerebral Palsy Registry Losing a diagnosis of cerebral palsy: a comparison of variables at 2 and 5 years. Dev. Med. Child Neurol. 62, 83–88 (2020).
Rosenbaum, P. et al. A report: the definition and classification of cerebral palsy April 2006. Dev. Med. Child Neurol. Suppl. 109, 8–14 (2007).
Richards, S. et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 17, 405–424 (2015).
Tavtigian, S. V. et al. Modeling the ACMG/AMP variant classification guidelines as a Bayesian classification framework. Genet. Med. 20, 1054–1060 (2018).
Backenroth, D. et al. CANOES: detecting rare copy number variants from whole exome sequencing data. Nucleic Acids Res. 42, e97 (2014).
Zare, F., Dow, M., Monteleone, N., Hosny, A. & Nabavi, S. An evaluation of copy number variation detection tools for cancer using whole exome sequencing data. BMC Bioinf. 18, 286 (2017).
Ellingford, J. M. et al. Validation of copy number variation analysis for next-generation sequencing diagnostics. Eur. J. Hum. Genet. 25, 719–724 (2017).
Liu, S. et al. Traditional karyotyping vs copy number variation sequencing for detection of chromosomal abnormalities associated with spontaneous miscarriage. Ultrasound Obstet. Gynecol. 46, 472–477 (2015).
Abyzov, A., Urban, A. E., Snyder, M. & Gerstein, M. CNVnator: an approach to discover, genotype, and characterize typical and atypical CNVs from family and population genome sequencing. Genome Res. 21, 974–984 (2011).
Dong, X. R. et al. Clinical exome sequencing as the first-tier test for diagnosing developmental disorders covering both CNV and SNV: a Chinese cohort. J. Med. Genet. 57, 558–566 (2020).
Aucott, S. W., Donohue, P. K. & Northington, F. J. Increased morbidity in severe early intrauterine growth restriction. J. Perinatol. 24, 435–440 (2004).
Molloy, E. J. et al. Neonatal encephalopathy and hypoxic-ischemic encephalopathy: moving from controversy to consensus definitions and subclassification. Pediatr. Res. 94, 1860–1863 (2023).
Jacobs, S. E. et al. Whole-body hypothermia for term and near-term newborns with hypoxic-ischemic encephalopathy: a randomized controlled trial. Obstet. Gynecol. Surv. 66, 743–744 (2011).
Azzopardi, D. V. et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N. Engl. J. Med. 361, 1349–1358 (2009).
Benders, M. J., Kersbergen, K. J. & de Vries, L. S. Neuroimaging of white matter injury, intraventricular and cerebellar hemorrhage. Clin. Perinatol. 41, 69–82 (2014).
Sibai, B. M. & Stella, C. L. Diagnosis and management of atypical preeclampsia-eclampsia. Am. J. Obstet. Gynecol. 200, e481–e487 (2009).
Acknowledgements
We thank B. Han and X. Chen from the Institute of Pediatrics, Children’s Hospital of Fudan University for their help with the nematode Caenorhabditis elegans experiment, and Y. Ping from the Bio-X Institutes, Key Laboratory for the Genetics of Developmental and Neuropsychiatric Disorders (Ministry of Education), Shanghai Jiao Tong University for his help in the fly Drosophila melanogaster experiment. We also thank the Core Facility of Shanghai Medical College, Fudan University for use of instruments. This work was supported by the Shanghai Municipal Commission of Science and Technology Research Project (19JC1411000 to Q.X.), the National Natural Science Foundation of China (U21A20347 to C. Zhu; 31972880, 32170615 and 31371274 to Q.X. and 82203969 to X.W.), the National Key Research and Development Program from the Ministry of Science and Technology of the People’s Republic of China (2021YFC2700800 to Q.X. and 2022YFC2704800 to C. Zhu), the National Key Research and Development Plan for Stem Cell and Translational Research (2017YFA0104202 to Q.X.), the collaborative innovation center project construction for Shanghai Women and Children’s Health (to Q.X.), a grant from Department of Science and Technology of Henan Province for international collaboration (GZS2023003 to X.W.), the Health Department of Henan Province (SBGJ202301009 to C. Zhu), Swedish governmental grants to scientists working in healthcare (ALFGBG-965197 to C. Zhu, ALFGBG-966034 to X.W.), the Swedish Research Council (2018-02267 and 2022-01019 to C. Zhu, and 2015-06276 and 2021-01950 to X.W.) and the Brain Foundation (FO2022-0120 to C. Zhu).
Author information
Authors and Affiliations
Contributions
Q.X., C. Zhu and X.W. were responsible for study conception, design, supervision and funding as well as analysis and interpretation of the data. Y.W., Y.C. and N.Q. were responsible for performing the ES, data acquisition, analysis and interpretation. Y.X., C. Zhou, Q.S., L.X., J.S., C.G., M.L. and D.Z. were responsible for cohort ascertainment, recruitment and phenotypic characterization. Y.W. drafted the manuscript and Q.X., C. Zhu, X.W., A.H.M., A.M.-D.-L. and J.G. revised the manuscript critically for important intellectual content. N.Q., Y.Q., X.Z., C.M., Y.F., X.P., S.W., N.L., B.L., Y. Sun, B.Z., T.L., H.L., J.Z., Y. Su, Q.L., J.Y., L.L. and D.Z. provided data, developed models, reviewed results and provided guidance on methods. All authors contributed and critically reviewed the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks David Amor, David Ledbetter and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Sonia Muliyil, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 The correlation between CP phenotypes and genetic findings.
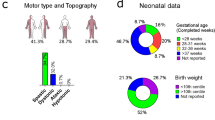
In order to determine the relationship between the different clinical phenotypes and the molecular diagnosis of individuals with CP, we performed clinical grouping of individuals, including classification, developmental profiles, and morphological examination. Global developmental delay/Intellectual Disability(GDD/ID), termed intellectual disability, was identified based on a score of less than 70 on the Bayley scales for measurement of the mental development index. The classification of CP shown in a includes groups with a large number of individuals: spastic quadriplegia, spastic diplegia, dyskinesia CP, and mixed types. The developmental profiles shown in b show complications of individuals with CP, such as speech disability. Shown in c are some imaging findings of CP, such as white matter injury and hydrocephalus. The results show that children with GDD/ID were closely related to heredity. The green is genetic diagnosis positive rate, and the gray is genetic diagnosis negative rate. The numbers on the top of the column represent the corresponding number of people. A two-side Fisher’s exact test was used in comparison of genetic diagnostic rate in specific CP subgroup. Bonferroni was used to handle multiple test problem. ‘*’ means P < 0.05, ‘**’ means P < 0.01, and ‘***’ means P < 0.001.
Extended Data Fig. 2 Phenotype detection of nematode motility development.
After the strains L4440 and Escherichia coli containing target gene RNAi were fed to rrf-3 mutant nematode and TU3311 strain, we measured and analyzed the body length, body width, moving distance and speed. The differences were compared using a statistical method called the Log-rank(Mantel-Cox) Test. n = 50 worms for each case. ‘*’ means P < 0.05, ‘**’ means P < 0.01, and ‘***’ means P < 0.001. Data are presented as mean values +/− SEM. a. Mean Worm Length (um): The body length showed no significant difference in nmpg-1 or tom-1 target gene RNAi bacteria feeding rrf-3 mutant nematode (P = 0.22 and 0.075, respectively), but the body length was increased when feeding TU3311 strain nematode (P = 0.044 and 0.0011 in TU3311). b. Mean Width (um): There was no significant difference in the body width of the mutant strains (P = 0.53 and 0.18 in rrf-3, and P = 0.72 and 0.10 in TU3311). c. Speed (um/s): When the tom-1 target gene RNAi bacteria were fed to rrf-3 mutant nematode, the movement speed was significantly slower than that of the nematode fed by L4440 bacteria (P = 0.0002). When feeding tom-1 target gene RNAi bacteria to TU3311 strain nematode, the movement speed was faster than that fed by L4440 strain nematode (P = 0.011). For nmgp-1 RNAi bacteria fed rrf-3 mutant nematode and TU3311 strain nematode, there was no significant difference (P = 0.88 and 0.21, respectively). d. Track Length (um): When feeding tom-1 target gene RNAi bacteria to rrf-3 nematode, the distance was shorter than that fed by L4440 bacteria (P = 0.0002). When feeding tom-1 target gene RNAi bacteria to TU3311 strain nematode, the track length was longer than that fed by L4440 strain nematode (P = 0.011). There was no significant difference in distance when feeding nmgp-1 target gene RNAi bacteria (P = 0.88 and 0.21 in rrf-3 and TU3311 strain, respectively).
Extended Data Fig. 3 Knockdown of Tom and Bsg affects larval and adult locomotion.
a. Relative crawling speed of a single 3rd instar larval was measured as the number of squares (sqr) crossed during test for each genotype as indicated (P = 0.019 and 0.0002 for Bsg knockdown). n = 15 larvae for each case. b. Adult climbing locomotion was measured as the time for 50% flies to reach the threshold line (8 cm from the bottom) for different genotypes as indicated (P = 0.0062 and 0.0010 for Tom knockdown; P = 0.0022 and 0.0043 for Bsg knockdown). n = 6 flies for each case. The differences were compared using a statistical method called the Log-rank (Mantel-Cox) Test. ‘*’ means P < 0.05, ‘**’ means P < 0.01, and ‘***’ means P < 0.001. Data are presented as mean values +/− SEM.
Extended Data Fig. 4 Filtering process for annotated files.
The screening procedure according to the ACMG for (likely) pathogenic variants through annotated files is shown for a single sample. After deletion of some sites like high-frequency sites and synonymous sites, the preliminary analysis of the sites was performed through prediction software scoring. The remaining variants were further divided into categories based on the literature. All variants were verified by Sanger sequencing.
Extended Data Fig. 5 The KEGG pathway of the P/LP-variants-related genes and VUS-related genes.
a showed the KEGG pathway using P/ LP-variants-related genes (n = 218). And b is the KEGG pathway using VUS-related genes (n = 299). The size of the circle represents the number of genes in the pathway. Hypergeometric test was used in the pathway enrichment analysis and Benjamin and Hochberg method was applied to deal with the multiple correction problem. The color is the corrected p-value, and the darker the color, the more significant the p-value.
Supplementary information
Supplementary Information
Supplementary Results and Methods.
Supplementary Tables
Supplementary Tables 1–8.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., Xu, Y., Zhou, C. et al. Exome sequencing reveals genetic heterogeneity and clinically actionable findings in children with cerebral palsy. Nat Med (2024). https://doi.org/10.1038/s41591-024-02912-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41591-024-02912-z