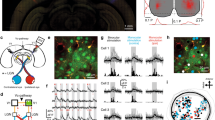
Abstract
Experience-dependent plasticity in the mature visual system is widely considered to be cortical. Using chronic two-photon Ca2+ imaging of thalamic afferents in layer 1 of binocular visual cortex, we provide evidence against this tenet: the respective dorsal lateral geniculate nucleus (dLGN) cells showed pronounced ocular dominance (OD) shifts after monocular deprivation in adult mice. Most (86%), but not all, of dLGN cell boutons were monocular during normal visual experience. Following deprivation, initially deprived-eye-dominated boutons reduced or lost their visual responsiveness to that eye and frequently became responsive to the non-deprived eye. This cannot be explained by eye-specific cortical changes propagating to dLGN via cortico-thalamic feedback because the shift in dLGN responses was largely resistant to cortical inactivation using the GABAA receptor agonist muscimol. Our data suggest that OD shifts observed in the binocular visual cortex of adult mice may at least partially reflect plasticity of eye-specific inputs onto dLGN neurons.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hubel, D. H. & Wiesel, T. N. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J. Physiol. (Lond.) 206, 419–436 (1970).
LeVay, S., Wiesel, T. N. & Hubel, D. H. The development of ocular dominance columns in normal and visually deprived monkeys. J. Comp. Neurol. 191, 1–51 (1980).
Sato, M. & Stryker, M. P. Distinctive features of adult ocular dominance plasticity. J. Neurosci. 28, 10278–10286 (2008).
Rose, T., Jaepel, J., Hübener, M. & Bonhoeffer, T. Cell-specific restoration of stimulus preference after monocular deprivation in the visual cortex. Science 352, 1319–1322 (2016).
Sawtell, N. B. et al. NMDA receptor-dependent ocular dominance plasticity in adult visual cortex. Neuron 38, 977–985 (2003).
Wiesel, T. N. & Hubel, D. H. Single-cell responses in striate cortex of kittens deprived of vision in one eye. J. Neurophysiol. 26, 1003–1017 (1963).
Dräger, U. C. Observations on monocular deprivation in mice. J. Neurophysiol. 41, 28–42 (1978).
Sherman, S. M. & Spear, P. D. Organization of visual pathways in normal and visually deprived cats. Physiol. Rev. 62, 738–855 (1982).
Wiesel, T. N. & Hubel, D. H. Effects of visual deprivation on morphology and physiology of cells in the cats lateral geniculate body. J. Neurophysiol. 26, 978–993 (1963).
Moore, B. D. IV, Kiley, C. W., Sun, C. & Usrey, W. M. Rapid plasticity of visual responses in the adult lateral geniculate nucleus. Neuron 71, 812–819 (2011).
Fox, K., Wallace, H. & Glazewski, S. Is there a thalamic component to experience-dependent cortical plasticity? Phil. Trans. R. Soc. Lond. B 357, 1709–1715 (2002).
Tian, N. & Copenhagen, D. R. Visual deprivation alters development of synaptic function in inner retina after eye opening. Neuron 32, 439–449 (2001).
Huberman, A. D., Feller, M. B. & Chapman, B. Mechanisms underlying development of visual maps and receptive fields. Annu. Rev. Neurosci. 31, 479–509 (2008).
Ziburkus, J. & Guido, W. Loss of binocular responses and reduced retinal convergence during the period of retinogeniculate axon segregation. J. Neurophysiol. 96, 2775–2784 (2006).
Chen, C. & Regehr, W. G. Developmental remodeling of the retinogeniculate synapse. Neuron 28, 955–966 (2000).
Jaubert-Miazza, L. et al. Structural and functional composition of the developing retinogeniculate pathway in the mouse. Vis. Neurosci. 22, 661–676 (2005).
Gilbert, C. D. & Wiesel, T. N. Receptive field dynamics in adult primary visual cortex. Nature 356, 150–152 (1992).
Grubb, M. S., Rossi, F. M., Changeux, J. P. & Thompson, I. D. Abnormal functional organization in the dorsal lateral geniculate nucleus of mice lacking the beta 2 subunit of the nicotinic acetylcholine receptor. Neuron 40, 1161–1172 (2003).
Zhao, X., Liu, M. & Cang, J. Sublinear binocular integration preserves orientation selectivity in mouse visual cortex. Nat. Commun. 4, 2088 (2013).
Grieve, K. L. Binocular visual responses in cells of the rat dLGN. J. Physiol. (Lond.) 566, 119–124 (2005).
Howarth, M., Walmsley, L. & Brown, T. M. Binocular integration in the mouse lateral geniculate nuclei. Curr. Biol. 24, 1241–1247 (2014).
Zeater, N., Cheong, S. K., Solomon, S. G., Dreher, B. & Martin, P. R. Binocular visual responses in the primate lateral geniculate nucleus. Curr. Biol. 25, 3190–3195 (2015).
Rompani, S. B. et al. Different modes of visual integration in the lateral geniculate nucleus revealed by single-cell-initiated transsynaptic tracing. Neuron 93, 767–776.e6 (2017).
Wiesel, T. N. Postnatal development of the visual cortex and the influence of environment. Nature 299, 583–591 (1982).
Kaneko, M., Stellwagen, D., Malenka, R. C. & Stryker, M. P. Tumor necrosis factor-alpha mediates one component of competitive, experience-dependent plasticity in developing visual cortex. Neuron 58, 673–680 (2008).
Stafford, B. K. & Huberman, A. D. Signal integration in thalamus: labeled lines go cross-eyed and blurry. Neuron 93, 717–720 (2017).
Chen, T.-W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010).
Cruz-Martín, A. et al. A dedicated circuit links direction-selective retinal ganglion cells to the primary visual cortex. Nature 507, 358–361 (2014).
Sun, W., Tan, Z., Mensh, B. D. & Ji, N. Thalamus provides layer 4 of primary visual cortex with orientation- and direction-tuned inputs. Nat. Neurosci. 19, 308–315 (2016).
Kondo, S. & Ohki, K. Laminar differences in the orientation selectivity of geniculate afferents in mouse primary visual cortex. Nat. Neurosci. 19, 316–319 (2016).
Roth, M. M. et al. Thalamic nuclei convey diverse contextual information to layer 1 of visual cortex. Nat. Neurosci. 19, 299–307 (2016).
Piscopo, D. M., El-Danaf, R. N., Huberman, A. D. & Niell, C. M. Diverse visual features encoded in mouse lateral geniculate nucleus. J. Neurosci. 33, 4642–4656 (2013).
Aarts, E., Verhage, M., Veenvliet, J. V., Dolan, C. V. & van der Sluis, S. A solution to dependency: using multilevel analysis to accommodate nested data. Nat. Neurosci. 17, 491–496 (2014).
Lur, G., Vinck, M. A., Tang, L., Cardin, J. A. & Higley, M. J. Projection-specific visual feature encoding by layer 5 cortical subnetworks. Cell Reports 14, 2538–2545 (2016).
Matthies, U., Balog, J. & Lehmann, K. Temporally coherent visual stimuli boost ocular dominance plasticity. J. Neurosci. 33, 11774–11778 (2013).
Greifzu, F. et al. Environmental enrichment extends ocular dominance plasticity into adulthood and protects from stroke-induced impairments of plasticity. Proc. Natl. Acad. Sci. USA 111, 1150–1155 (2014).
Bickford, M. E. Thalamic circuit diversity: modulation of the driver/modulator framework. Front. Neural Circuits 9, 86 (2016).
Denman, D. J. & Contreras, D. Complex effects on in vivo visual responses by specific projections from mouse cortical layer 6 to dorsal lateral geniculate nucleus. J. Neurosci. 35, 9265–9280 (2015).
Usrey, W. M. & Alitto, H. J. Visual functions of the thalamus. Annu. Rev. Vis. Sci. 1, 351–371 (2015).
Scholl, B., Tan, A. Y. Y., Corey, J. & Priebe, N. J. Emergence of orientation selectivity in the Mammalian visual pathway. J. Neurosci. 33, 10616–10624 (2013).
Wekselblatt, J. B., Flister, E. D., Piscopo, D. M. & Niell, C. M. Large-scale imaging of cortical dynamics during sensory perception and behavior. J. Neurophysiol. 115, 2852–2866 (2016).
Yamauchi, T., Hori, T. & Takahashi, T. Presynaptic inhibition by muscimol through GABAB receptors. Eur. J. Neurosci. 12, 3433–3436 (2000).
Coleman, J. E., Law, K. & Bear, M. F. Anatomical origins of ocular dominance in mouse primary visual cortex. Neuroscience 161, 561–571 (2009).
Baden, T. et al. The functional diversity of retinal ganglion cells in the mouse. Nature 529, 345–350 (2016).
Krahe, T. E. & Guido, W. Homeostatic plasticity in the visual thalamus by monocular deprivation. J. Neurosci. 31, 6842–6849 (2011).
Hooks, B. M. & Chen, C. Distinct roles for spontaneous and visual activity in remodeling of the retinogeniculate synapse. Neuron 52, 281–291 (2006).
Morgan, J. L., Berger, D. R., Wetzel, A. W. & Lichtman, J. W. The fuzzy logic of network connectivity in mouse visual thalamus. Cell 165, 192–206 (2016).
Hammer, S., Monavarfeshani, A., Lemon, T., Su, J. & Fox, M. A. Multiple retinal axons converge onto relay cells in the adult mouse thalamus. Cell Reports 12, 1575–1583 (2015).
Chen, C., Bickford, M. E. & Hirsch, J. A. Untangling the web between eye and brain. Cell 165, 20–21 (2016).
Durand, S. et al. A comparison of visual response properties in the lateral geniculate nucleus and primary visual cortex of awake and anesthetized mice. J. Neurosci. 36, 12144–12156 (2016).
Morin, L. P. & Studholme, K. M. Retinofugal projections in the mouse. J. Comp. Neurol. 522, 3733–3753 (2014).
Harrington, M. E. The ventral lateral geniculate nucleus and the intergeniculate leaflet: interrelated structures in the visual and circadian systems. Neurosci. Biobehav. Rev. 21, 705–727 (1997).
Charbonneau, V., Laramée, M.-E., Boucher, V., Bronchti, G. & Boire, D. Cortical and subcortical projections to primary visual cortex in anophthalmic, enucleated and sighted mice. Eur. J. Neurosci. 36, 2949–2963 (2012).
Hofer, S. B., Mrsic-Flogel, T. D., Bonhoeffer, T. & Hübener, M. Prior experience enhances plasticity in adult visual cortex. Nat. Neurosci. 9, 127–132 (2006).
Pologruto, T. A., Sabatini, B. L. & Svoboda, K. ScanImage: flexible software for operating laser scanning microscopes. Biomed. Eng. Online 2, 13 (2003).
Pelli, D. G. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat. Vis. 10, 437–442 (1997).
Brainard, D. H. The Psychophysics Toolbox. Spat. Vis. 10, 433–436 (1997).
Liu, B. H., Wu, G. K., Arbuckle, R., Tao, H. W. & Zhang, L. I. Defining cortical frequency tuning with recurrent excitatory circuitry. Nat. Neurosci. 10, 1594–1600 (2007).
Khibnik, L. A., Cho, K. K. & Bear, M. F. Relative contribution of feedforward excitatory connections to expression of ocular dominance plasticity in layer 4 of visual cortex. Neuron 66, 493–500 (2010).
Mazurek, M., Kager, M. & Van Hooser, S. D. Robust quantification of orientation selectivity and direction selectivity. Front. Neural Circuits 8, 92 (2014).
Petreanu, L. et al. Activity in motor-sensory projections reveals distributed coding in somatosensation. Nature 489, 299–303 (2012).
Acknowledgements
We thank C. Huber, V. Staiger, F. Voss and H. Tultschin for technical assistance, J. Sigl-Glöckner and D. Laubender for help with our transfection protocol, and P. Goltstein for programming help. This work was supported by the Max Planck Society, a Marie Curie Intra-European Fellowship to T.R., a Boehringer Ingelheim Ph.D. fellowship to J.J., and the Deutsche Forschungsgemeinschaft (grant SFB870 to T.R., M.H. and T.B.).
Author information
Authors and Affiliations
Contributions
J.J. and T.R. performed the experiments and analyzed the data. J.J., M.H., T.B. and T.R. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 dLGN virus injections and matching of regions of interest (ROIs) across sessions
a) AAV coding for GCaMP6m was injected into the dLGN of Scnn1A-Tg3-Cre mice expressing Cre-recombinase in cortical layer 4 and thalamus. The representative coronal section shows GCaMP6m-labeled neurons in the dLGN (outlined in right panel, scale bar: left 500 μm, right: 200 μm). Note absence of transfected cells in the injection path and negligible dorsomedial spread into the lateral posterior nucleus (LP). b) Pairwise signal correlation of all boutons belonging to the same morphologically identified axon (blue) in comparison to pairwise correlation between all boutons (red, see62 for a similar quantification). Triangles indicate median correlation coefficients (n = 4 field of views of 4 randomly selected animals). Signal correlations were defined as Pearson’s correlation coefficient between response vectors of concatenated mean responses to stimulation of either eye. c) Example image volumes illustrating matching of bouton ROIs across sessions. Changes in tissue morphology and positioning inaccuracies can result in individual boutons being optimally sectioned by different image planes at different time points (arrows of the same color indicate ROIs of the same group, image plane depth increment: 3 μm, first frame of volume 20 µm below pial surface; scale bar: 10 μm)
Supplementary Figure 2 Spatial receptive field properties of thalamic afferents in layer 1 of V1
a) Example Ca2+ traces (average of 10 trials) of a single bouton responding to pseudorandomized sparse noise stimulation (white, left, and black, right, patches on an isoluminant gray background; blue lines indicate time of stimulus presentation) of the contralateral eye, ordered according to stimulus position (scale bars: ΔF/F0 = 50%, 1 s). b) Receptive field of the corresponding bouton in (a). Top plots show interpolation of the raw ON (blue) and OFF (purple) receptive sub-fields at 1° resolution. Bottom plot shows the thresholded outline of the ON and OFF sub-fields (light: ON, dark: OFF). c) Violin and overlaid box-plots of spatial receptive field size of dLGN boutons and excitatory layer 2/3 cell bodies in V1 to ipsilateral (red) or contralateral (blue) eye stimulation, respectively. In each box-plot, the central mark indicates the median, and the bottom and top edges of the box indicate the 25th and 75th percentiles, respectively. The whiskers extend to the most extreme data points not considered outliers (1.5 times the interquartile range). The shaded areas in the violin plots show the mirrored and normalized probability density estimates (dLGN, n = 2658 receptive fields, 15 mice; V1, n = 123 receptive fields, 4 mice; combined median receptive field area dLGN: 195 ± 86 deg²; V1: 208 ± 99 deg², Z(1, 2779) = 1.05, P = 0.29; Wilcoxon rank sum). d) Distribution of all dLGN bouton receptive field centers from all mice across the visual field, color coded by the number of boutons per sector (n = 2658 receptive fields, 15 mice)
Supplementary Figure 3 Population orientation-selectivity, direction-selectivity and eye-specific responsiveness of thalamic projections to layer 1 of V1
a) Ca2+ signals of three orientation-selective example boutons in response to ipsi- and contralateral eye drifting grating stimulation (scale bars: ΔF/F0 = 200%, 10 s; gray traces: individual trials; black traces: mean response; red and blue bars: time of stimulus presentation). b) Polar plots displaying response amplitude (in ΔF/F0) of the boutons shown in (a). Numbers indicate global orientation selectivity indices (gOSI = 1 – circular variance). c, d) Fraction of orientation- (c, gOSI > 0.33) and direction-selective (d, gDSI > 0.33) ipsi-, contralateral and binocular dLGN boutons and V1 layer 2/3 cell bodies, respectively (dLGN, n = 21 mice; V1, n = 14 mice; all gOSI dLGN vs. V1, Z(2,33) = 8.0, P < 10-16; all gDSI dLGN vs. V1, Z(2,33) = 8.1, P < 10-16; Wilcoxon rank sum test; gOSI dLGN, χ2(2) = 1.5, P = 0.47; gDSI dLGN, χ2(2) = 0, P = 0.98; gOSI V1, χ2(2) = 3.2, P = 0.20; gDSI V1, χ2(2) = 4.0, P = 0.14; Kruskal-Wallis test). e, f) Weighted mean gOSI (e) and gDSI (f) (error bars: SD) of ipsilateral, contralateral and binocular dLGN boutons and layer 2/3 cell bodies, respectively, after multilevel analysis (dLGN, n = 21 mice; V1, n = 14 mice; all gOSI dLGN vs. V1, t(4066) = -48.4, P = 0; all gDSI dLGN vs. V1, t(4066) = -35.5, P = 0; t-test; gOSI dLGN: F(2,1970) = 4.2, P = 0.015; ipsi vs. contra, P = 0.33; ipsi vs. binocular, P = 0.015; contra vs. binocular, P = 0.16; gDSI dLGN: F(2,1970) = 4.7, P = 0.009; ipsi vs. contra, P = 1; ipsi vs. binocular, P = 0.27; contra vs. binocular, P = 0.03; gOSI V1: F(2,2092) = 14.00, P < 10-6; ipsi vs. contra, P < 10-6; ipsi vs. binocular, P < 10-4; contra vs. binocular, P = 0.73; gDSI V1: F(2,2092) = 7.4, P < 10-4; ipsi vs. contra, P = 0.02; ipsi vs. binocular, P = 1; contra vs. binocular, P = 0.0015; one-way ANOVA, Bonferroni corrected. g) Peak-normalized contra- (blue) and ipsilalateral (red) population response of dLGN boutons (left traces) and excitatory layer 2/3 cell bodies in V1 (right traces; mean ± SEM response to preferred drifting grating stimulation, normalized to contralateral response, dashed lines; black lines: stimulus presentation, scale bars: 5 s, 0.25 arbitrary units, a.u.; dLGN, contra/ipsi ratio = 2.04, n = 1973 boutons; V1, contra/ipsi ratio = 1.86, n = 2095 cells). Arrowhead indicates the pronounced dLGN on-response to the change in luminance evoked by pseudorandom eye-shutter switches, which is absent in cortex. h) Population contra/ipsi ratio in dLGN and V1 (dLGN, n = 21 mice; V1, n = 14 mice; Z(2,33) = -0.89, P = 0.37; Wilcoxon rank sum test). Layer 2/3 data reanalyzed from Rose et al. 20164
Supplementary Figure 4 Stability of eye-specific responses during normal visual experience
a) Eye-specific fluorescence changes in response to drifting grating stimulation (8 directions) in 1498 dLGN boutons in twelve animals during baseline. Horizontally, responses are sorted for preferred direction and vertically by contralateral eye response magnitude in the first session, only (as indicated by black wedges; blue and red bars: time of stimulus presentation). The same vertical sorting is used throughout such that rows correspond to the same recorded bouton. b) Quantification of bouton-wise mean changes in eye-specific response amplitude during baseline, grouped into pre-MD ODI sextiles comprising similar numbers of boutons (n = 530 boutons responsive in all pre-MD sessions, 87-89 boutons per class; mean (black lines) ± SEM (gray shading); monocular ipsilateral and contralateral ODI classes indicated by red and blue shading as indicated and defined in Fig. 2c,g). c) ODI of boutons during baseline (n = 578 boutons from 12 mice, χ2(2) = 20.6, P < 10-4; session 1 vs. session 2, P < 10-4; session 2 vs. session 3, P = 0.002; Friedman test, Bonferroni corrected)
Supplementary Figure 5 OD plasticity and recovery from OD plasticity in thalamic afferents
a) Schematic of stimulus presentation for intrinsic optical imaging. Visual stimuli were presented at two different locations on a screen centered on the binocular visual field of the mouse. b) Color-coded maps (see a) of cortical responses to stimulation of the ipsilateral (top panel) or contralateral (bottom panel) eye (scale bar: 0.5 mm), overlaid on an image of the cortical surface blood vessel pattern. c) ODI values based on intrinsic optical imaging of cortical responses for normal adult mice (mean ± SEM: 0.27 ± 0.04, n = 12), after 6-8 days of MD (-0.06 ± 0.07, n = 12), and after 1-2 weeks of recovery (0.15 ± 0.05, n = 6 animals, χ2(2) = 22.3, P < 10-5; Kruskal-Wallis test, Bonferroni corrected, baseline vs. MD, P < 10-5; baseline vs. recovery, P = 0.22; MD vs. recovery, P = 0.41). d) Example time course of dLGN single-bouton ODI (gray lines) from one animal over baseline, contralateral eye MD and recovery (black line: mean ODI). Conventions for sessions quantified are indicated on top (mean pre-MD, pre-MD, post-MD, and recovery). e) OD Shift magnitude of boutons with receptive field size < 200deg2. Single-bouton ODI distribution during baseline (mean of three pre-MD sessions, ODI = 0.35 ± 0.67, standard deviation, SD) and after 6-8 days of MD (post-MD: ODI = 0.20 ± 0.58, SD, n = 78 boutons, 5 animals, Z(77) = 2.6, P = 0.01, Wilcoxon signed-rank test, compare Fig. 2c). Lines connect individual, continuously responsive boutons; line color indicates shift significance in units of standard deviations over pre-MD baseline fluctuations. Colored ODI histogram bins indicate class definitions for contralateral (blue), binocular (white) and ipsilateral (red) boutons (see Fig 2c). ( f) Eye-specific fluorescence changes in response to drifting grating stimulation (8 directions) in 1507 dLGN boutons in 12 animals before and after MD. All responses are sorted for preferred direction (horizontally) and contralateral or ipsilateral eye response magnitude (vertically) in each session (as indicated by the black wedges; blue and red bars: time of stimulus presentation). Note the decrease in contra- and increase in ipsilateral eye responsiveness. g) Mean ODI ( ± SEM) of boutons (n = 132 boutons from 5 mice, responsive during baseline, MD and recovery) after MD and after at least one week of binocular vision (χ2(2) = 59.3, P < 10-12; baseline vs. MD, P < 10-7; MD vs. recovery, P < 10-11; baseline vs. recovery, P = 0.47; Friedman test, Bonferroni corrected). Lines here and in (h,i) connect individual, continuously responsive boutons. h) Mean response amplitude ( ± SEM) to ipsilateral eye stimulation after MD and at least one week of binocular vision (χ2(2) = 59.3, P < 10-10; baseline vs. MD, P < 10-5; MD vs. recovery, P < 10-10; baseline vs. recovery, P = 0.25; Friedman test, Bonferroni corrected). i) Mean response amplitude ( ± SEM) to contralateral eye stimulation after MD and at least one week of binocular vision (χ2(2) = 11.2, P = 0.004; baseline vs. MD, P = 0.003; MD vs. recovery, P = 0.07; baseline vs. recovery, P = 0.97; Friedman test, Bonferroni corrected)
Supplementary Figure 6 OD plasticity in excitatory layer 2/3 cells in V1
a) Eye-specific fluorescence changes in response to drifting grating stimulation (8 directions) in 1335 layer 2/3 cells in eight animals before and after MD. Horizontally, responses are sorted for preferred direction and vertically by eye-specific response magnitude in each session (as indicated by the black wedges; blue and red bars: time of stimulus presentation). Note the decrease in contra- and increase in ipsilateral eye responsiveness. Here and in d) we quantified changes in the fluorescence ratio (R) of the GCaMP6s signal over a red structural marker4. b) ODI distribution of the same excitatory layer 2/3 cells in V1 during baseline (mean of 3 pre-MD sessions, 0.28 ± 0.47, SD) and after 5-8 days of MD (0.07 ± 0.67, SD, n = 430 cells in 10 mice, Z(429) = 6.7, P < 10-10; Wilcoxon signed-rank test). Lines connect individual, continuously responsive cells; line color indicates shift significance in units of standard deviations over pre-MD baseline fluctuations. Colored ODI histogram bins indicate class definitions for contralateral (blue), binocular (white) and ipsilateral (red) cells (see Fig. 2c; data reanalyzed from Rose et al. 20164). c) Cumulative eye-specific response amplitude before and after MD (n = 1635 layer 2/3 cells, re-found before and after MD, ipsilateral eye responses baseline vs. post-MD, P < 10-4; contralateral eye responses baseline vs. post-MD, P < 10-13; two-sample Kolmogorov-Smirnov tests). d) Quantification of cell-wise change in eye-specific response amplitude after MD, grouped into pre-MD ODI sextiles comprising similar numbers of cells (n = 565 cells responsive in all pre-MD sessions 10 animals; 93-94 cells per class; mean (black lines) ± SEM (gray shading); paired t-tests; monocular ODI classes indicated by red and blue shading as defined in (b) and Fig. 2c, g)
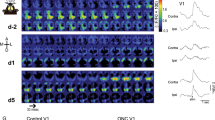
Supplementary Figure 7 Silencing of visual cortex using muscimol applied to the intact dura
a/c) Activity maps (maximum projections over 13 min of recording) of the same field of view during visual stimulation before and after muscimol application in layer 2/3 (a) and layer 5/6 cells (c) in visual cortex of GCaMP6 animals (CamKIIa-tTa;Tg(tetO-GCaMP6s)2Niell). Overlay: Contra- (blue) and ipsilalateral (red) population responses (mean ± SEM) of layer 2/3 cells as well as binocular (gray) population response of layer 5/6 cells in V1 in response to preferred drifting grating stimulation (white bars: time of stimulus presentation). Note lack of responses ~2h after topical application of muscimol. b) Median change in Ca2+ signal in response to visual stimulation of the ipsilateral or contralateral eye, respectively, in layer 2/3 cells. On each box-plot here and below, the central mark indicates the median, and the bottom and top edges of the box indicate the 25th and 75th percentiles, respectively. The whiskers extend to the most extreme data points not considered outliers (1.5 times the interquartile range, outliers not shown; 2 mice, n = 62 cells; ipsilateral eye response, Z(61) = 6.7, P < 10-10; contralateral eye response, Z(61) = 6.7, P < 10-10; Wilcoxon signed-rank tests. d) Median response amplitudes to binocular stimulation before and after muscimol application recorded at a depth of 590-650 µm below dura (putatively deep layer 5, upper layer 6; 3 mice; pre-muscimol, n = 139 cells, post-muscimol, n = 120 cells; Z(1, 257) = 12.6, P = 0, Wilcoxon rank sum test). e) V1 activation, as measured with intrinsic optical imaging, evoked by stimulation of the contralateral (blue) or ipsilateral (red) eye before and after muscimol application (n = 2 mice, mean ± SEM). f) Median response amplitude to visual stimulation after application of the GABA-A agonist muscimol together with the GABA-B antagonist SCH50911 of thalamic boutons in layer 1 of V1 in wildtype mice (n = 251 boutons in 3 animals, ipsilateral eye responses, Z(250) = 4.2, P = 0.33; contralateral eye responses, Z(250) = 1.0, P < 10-4; Wilcoxon signed-rank)
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7
Rights and permissions
About this article
Cite this article
Jaepel, J., Hübener, M., Bonhoeffer, T. et al. Lateral geniculate neurons projecting to primary visual cortex show ocular dominance plasticity in adult mice. Nat Neurosci 20, 1708–1714 (2017). https://doi.org/10.1038/s41593-017-0021-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-017-0021-0
This article is cited by
-
Induction of excitatory brain state governs plastic functional changes in visual cortical topology
Brain Structure and Function (2023)
-
Host interneurons mediate plasticity reactivated by embryonic inhibitory cell transplantation in mouse visual cortex
Nature Communications (2021)
-
Mouse visual cortex areas represent perceptual and semantic features of learned visual categories
Nature Neuroscience (2021)
-
The Visual Pathway for Binocular Integration
Neuroscience Bulletin (2020)
-
Therapeutic effect of vasoactive intestinal peptide on form-deprived amblyopic kittens
BMC Ophthalmology (2019)