Abstract
Excitation of accumbal D2 cells governs vital actions, including avoidance of learned risks, but the origins of this excitation and roles of D2 cells in innate risk-avoidance are unclear. Hypothalamic neurons producing orexins (also called hypocretins) enhance innate risk-avoidance via poorly understood neurocircuits. We describe a direct orexin→D2 excitatory circuit and show that D2 cell activity is necessary for orexin-dependent innate risk-avoidance in mice, thus revealing an unsuspected hypothalamus–accumbens interplay in action selection.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
05 June 2018
In the version of this article initially published, a sentence in the third paragraph read, “Suppression of natural orexin signaling by orexin receptor antagonism (Fig. 3b–e) led to increased risk-avoidance behaviors.” “Increased” has been changed to “decreased” in this sentence. The error has been corrected in the HTML and PDF versions of the article.
References
Sakurai, T. Nat. Rev. Neurosci. 15, 719–731 (2014).
Giardino, W. J. & de Lecea, L. Curr. Opin. Neurobiol. 29, 103–108 (2014).
González, J. A., Iordanidou, P., Strom, M., Adamantidis, A. & Burdakov, D. Nat. Commun. 7, 11395 (2016).
Peyron, C. et al. J. Neurosci. 18, 9996–10015 (1998).
Maner, J. K. & Schmidt, N. B. Behav. Ther. 37, 181–189 (2006).
Johnson, P. L. et al. Nat. Med. 16, 111–115 (2010).
Suzuki, M., Beuckmann, C. T., Shikata, K., Ogura, H. & Sawai, T. Brain Res. 1044, 116–121 (2005).
Bonnavion, P., Jackson, A. C., Carter, M. E. & de Lecea, L. Nat. Commun. 6, 6266 (2015).
Heydendael, W., Sengupta, A., Beck, S. & Bhatnagar, S. Physiol. Behav. 130, 182–190 (2014).
Zalocusky, K. A. et al. Nature 531, 642–646 (2016).
Nicola, S. M., Surmeier, J. & Malenka, R. C. Annu. Rev. Neurosci. 23, 185–215 (2000).
Kupchik, Y. M. et al. Nat. Neurosci. 18, 1230–1232 (2015).
Koós, T. & Tepper, J. M. Nat. Neurosci. 2, 467–472 (1999).
Harris, G. C., Wimmer, M. & Aston-Jones, G. Nature 437, 556–559 (2005).
Mahler, S. V., Moorman, D. E., Smith, R. J., James, M. H. & Aston-Jones, G. Nat. Neurosci. 17, 1298–1303 (2014).
Gangarossa, G. et al. Front. Neural Circuits 7, 22 (2013).
Mori, A., Shindou, T., Ichimura, M., Nonaka, H. & Kase, H. J. Neurosci. 16, 605–611 (1996).
Matsuki, T. et al. Proc. Natl. Acad. Sci. USA 106, 4459–4464 (2009).
Schöne, C. et al. J. Neurosci. 32, 12437–12443 (2012).
Apergis-Schoute, J. et al. J. Neurosci. 35, 5435–5441 (2015).
Planert, H., Berger, T. K. & Silberberg, G. PLoS One 8, e57054 (2013).
Gertler, T. S., Chan, C. S. & Surmeier, D. J. J. Neurosci. 28, 10814–10824 (2008).
Kawaguchi, Y. J. Neurosci. 13, 4908–4923 (1993).
Kawaguchi, Y., Wilson, C. J., Augood, S. J. & Emson, P. C. Trends Neurosci. 18, 527–535 (1995).
Saddoris, M. P., Cacciapaglia, F., Wightman, R. M. & Carelli, R. M. J. Neurosci. 35, 11572–11582 (2015).
Alderson, H. L., Parkinson, J. A., Robbins, T. W. & Everitt, B. J. Psychopharmacology (Berl.) 153, 455–463 (2001).
van der Plasse, G., Schrama, R., van Seters, S. P., Vanderschuren, L. J. & Westenberg, H. G. PLoS One 7, e33455 (2012).
Vélez-Fort, M. et al. Neuron 83, 1431–1443 (2014).
Wickersham, I. R. et al. Neuron 53, 639–647 (2007).
Wall, N. R., Wickersham, I. R., Cetin, A., De La Parra, M. & Callaway, E. M. Proc. Natl. Acad. Sci. USA 107, 21848–21853 (2010).
Schöne, C., Apergis-Schoute, J., Sakurai, T., Adamantidis, A. & Burdakov, D. Cell Rep. 7, 697–704 (2014).
Blomeley, C. P., Cains, S. & Bracci, E. Front. Cell. Neurosci. 9, 453 (2015).
Smart, D. et al. Br. J. Pharmacol. 132, 1179–1182 (2001).
Adamantidis, A. R., Zhang, F., Aravanis, A. M., Deisseroth, K. & de Lecea, L. Nature 450, 420–424 (2007).
Karnani, M. M. et al. Neuron 72, 616–629 (2011).
Prut, L. & Belzung, C. Eur. J. Pharmacol. 463, 3–33 (2003).
Walsh, R. N. & Cummins, R. A. Psychol. Bull. 83, 482–504 (1976).
Dent, C. L., Isles, A. R. & Humby, T. Eur. J. Neurosci. 39, 520–530 (2014).
Fendt, M., Endres, T., Lowry, C. A., Apfelbach, R. & McGregor, I. S. Neurosci. Biobehav. Rev. 29, 1145–1156 (2005).
Yang, H. et al. Nat. Neurosci. 19, 283–289 (2016).
Whiddon, B. B. & Palmiter, R. D. J. Neurosci. 33, 2009–2016 (2013).
González, J. A. et al. Curr. Biol. 26, 2486–2491 (2016).
Acknowledgements
This work was funded by The Francis Crick Institute, which receives its core funding from Cancer Research UK, the UK Medical Research Council and the Wellcome Trust.
Author information
Authors and Affiliations
Contributions
C.B. and C.G. (equal contributors) designed and performed experiments and analyzed data; D.B. designed experiments, obtained funding and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated Supplementary Information
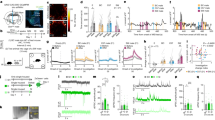
Supplementary Figure 1 Supplementary optogenetic and electrophysiological data
A. Studying electrical properties of NAc D2 neurons. Top: recording schematic. Bottom: classical electrophysiological dynamics (responses to current injections, purple) used to identify NAc D2R neurons (performed in every cell studied). B. Left: effect of bath-application of dopamine (40 mM) on D2 cell membrane potential responses to excitatory current injection (purple). Representative example of n = 8/8 cells (all cells responded). Right: group data (means±sem) illustrating inhibition of the evoked spiking for dopamine, n = 8 cells. C. Top: targeting scheme for studying responses of NAc NPY interneurons to photo-stimulation of LH orexin cells. Bottom: Expression of ChR2 in the LH (typical example of n = 5 brains). D. Top: recording schematic for studying LH orexin-->NAc NPY circuit. Bottom: confirmation that NAc NPY-eGFP neurons display defining electrical signatures of NAc NPY interneurons (response to current injection, purple, performed in every cell studied). E. Effect of orexin axon photostimulation on NAc NPY interneuron membrane potential (left) and current (right), in the presence of orexin receptor antagonists TCS/SB (see Supplementary Methods). Representative responses of n = 11 cells. F. Effect of low-frequency (1 Hz) orexin cell stimulation of NAc D2 cells (individual flash is shown here; this effect is representative of all flashes in the 1 Hz 10 s train), revealing a low-probability (n = 13/64 cells), CNQX-sensitive input.
Supplementary Figure 2 Supplementary data relating to cell-type-specific LH→NAc functional connectivity mapping
A, B. Probing orexin cell→D1 cell interaction. A,Targeting strategy (top) and resulting expression of mCherry (red) in LH (bottom), representative example of n = 5 brains showing expression localization typical of orexin cells. B, Recording scheme (top), and effects of low and high frequency orexin axon photo-stimulation on D1 cells (representative example of n = 48 neurons, 48/48 cells showed no detectable responses). C, D. Probing MCH→D2 cell interaction. C, Targeting strategy (top) and resulting expression of mCherry (red) in LH (bottom), representative example of n = 5 brains showing expression localization typical of MCH cells. D, Recording scheme (top), and effects of low and high frequency MCH axon photo-stimulation on D2 cells (representative example of n = 24 neurons, 24/24 cells showed no detectable responses).
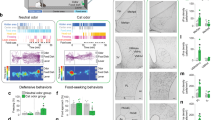
Supplementary Figure 3 Probing sufficiency of NAc orexin for innate risk-avoidance behaviors
A. Experimental paradigm: orexin or control vehicle solutions were infused bilaterally into accumbens via implanted cannulae, followed by behavioral assays, see Supplementary Methods. B. Effect of orexin on center preference in the open-field test. Left and center, examples of spatial exploration heatmaps from the same mouse infused with orexin or vehicle solutions. Right, effect of orexin or vehicle infusion on centre occupancy (% time during the first 15 min of arena exploration) in n = 10 mice. P value is from a two-tailed paired t test, t = 3.545 df = 9. In the same tests, orexin did not significantly affect locomotion velocity (vehicle = 7.24+/-3.5 cm/sec; orexin = 6.04+/-0.96 cm/s; p = 0.176 by two-tailed paired t-test, t=1.468 df=9). C. Effect of orexin on hesitation in the PORT test. Left and center, examples of spatial exploration heatmaps from the same mouse infused with orexin or vehicle solutions. Right, effect of orexin or vehicle infusion on hesitation to entry of middle (odor) chamber in n = 10 mice. P value is from a two-tailed paired t test, t = 4.281 df = 9.
Supplementary Figure 4 Effect of NAc D2 cell chemogenetic excitation on innate risk-avoidance behavior, and control experiments ruling out locomotor sedation as a confounding factor in open field experiments
A. Left, targeting scheme for hM3Dq-mCherry; Right, localization of hM3Dq –mCherry (red); ac = anterior commissure; representative example of n = 4 brains. B. Effect of CNO on membrane potential of a D2–hM3Dq cell (representative example of n = 10 cells; mean+/-sem depolarization = 7.92+/-0.91 mV, p < 0.0001 by two-tailed one-sample t-test; t,df=8.666,9). C. Example of spatial exploration heatmaps from CNO-injected mice expressing ChR2 or hM3Dq in NAc D2 cells. D. Effect of CNO on centre occupancy (% time during the first 15 min of arena exploration) in mice expressing hM3Dq or ChR2 in NAc D2 cells. 4 mice per group, values are individual mice and means ± sem. Two-way repeated measures ANOVA F(1,6) = 9.715, p = 0.0207; numbers above bars are p-values from Sidak’s post tests (ns = > 0.2). E. Effect on drug treatments on measures of sedation (locomotion velocity and immobility) in the open field test used to assess center-border preference. n = 8 mice in each group, values are individual mice and means ± sem. No significant effects were found: Right plot, two-way repeated-measures ANOVA F(3,42) = 0.2342, p = 0.872 (ns); Left plot, two-way repeated-measures ANOVA F(3,42) = 0.6046, p = 0.616 (ns).
Supplementary information
Supplementary Figures 1–4
Supplementary Text and Figures
Rights and permissions
About this article
Cite this article
Blomeley, C., Garau, C. & Burdakov, D. Accumbal D2 cells orchestrate innate risk-avoidance according to orexin signals. Nat Neurosci 21, 29–32 (2018). https://doi.org/10.1038/s41593-017-0023-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-017-0023-y
This article is cited by
-
Optogenetic and pharmacological interventions link hypocretin neurons to impulsivity in mice
Communications Biology (2023)
-
Activation of the rostral nucleus accumbens shell by optogenetics induces cataplexy-like behavior in orexin neuron-ablated mice
Scientific Reports (2023)
-
NAc-VTA circuit underlies emotional stress-induced anxiety-like behavior in the three-chamber vicarious social defeat stress mouse model
Nature Communications (2022)
-
A genetically encoded sensor for in vivo imaging of orexin neuropeptides
Nature Methods (2022)
-
A new GABAergic somatostatin projection from the BNST onto accumbal parvalbumin neurons controls anxiety
Molecular Psychiatry (2021)