Abstract
We identify a memory-specific attention mechanism in the human anterior temporal lobe, an area implicated in semantic processing and episodic memory formation. Spiking neuron activity is suppressed and becomes more reliable in preparation for verbal memory formation. Intracranial electroencephalography signals implicate this region as a source of executive control for attentional selection. Consistent with this interpretation, its surgical removal causes significant memory impairment for attended words relative to unattended words.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
20 August 2018
In the version of this article originally published, the reference citations in the Methods section were misnumbered. This has now been corrected in the HTML and PDF versions of the paper.
References
Chun, M. M., Golomb, J. D. & Turk-Browne, N. B. Annu. Rev. Psychol. 62, 73–101 (2011).
Cohen, M. R. & Maunsell, J. H. Nat. Neurosci. 12, 1594–1600 (2009).
Baldauf, D. & Desimone, R. Science 344, 424–427 (2014).
Hopfinger, J. B., Buonocore, M. H. & Mangun, G. R. Nat. Neurosci. 3, 284–291 (2000).
Maunsell, J. H. R. Annu. Rev. Vis. Sci. 1, 373–391 (2015).
Ruff, D. A. & Cohen, M. R. Nat. Neurosci. 17, 1591–1597 (2014).
Zar, J.H. Biostatistical Analysis (Pearson Education Inc, 2010), 5th edn.
Brown, P. et al. J. Neurosci. 21, 1033–1038 (2001).
Kahana, M. J. Foundations of Human Memory (Oxford University Press, New York, 2014).
Gazzaley, A. & Nobre, A. C. Trends Cogn. Sci. 16, 129–135 (2012).
Hautus, M. J. Behav. Res. Methods Instrum. Comput. 27, 46–51 (1995).
Haque, R., Wittig, J. Jr., Damera, S., Inati, S. K. & Zaghloul, K. A. J. Neurosci. 35, 13577–13586 (2015).
Trotta, M. S. et al. Hum. Brain Mapp. 39, 709–721 (2017).
Oosterhof, N. N., Wiestler, T., Downing, P. E. & Diedrichsen, J. Neuroimage 56, 593–600 (2011).
Burke, J. F., Ramayya, A. G. & Kahana, M. J. Curr. Opin. Neurobiol. 31, 104–110 (2015).
Manning, J. R., Jacobs, J., Fried, I. & Kahana, M. J. J. Neurosci. 29, 13613–13620 (2009).
Jang, A. I., Wittig, J. H. Jr., Inati, S. K. & Zaghloul, K. A. Curr. Biol. 27, 1700–1705.e5 (2017).
Yaffe, R. B. et al. Proc. Natl. Acad. Sci. USA 111, 18727–18732 (2014).
Joshua, M., Elias, S., Levine, O. & Bergman, H. J. Neurosci. Methods 163, 267–282 (2007).
Averbeck, B. B., Latham, P. E. & Pouget, A. Nat. Rev. Neurosci. 7, 358–366 (2006).
Fukushima, M., Saunders, R. C., Leopold, D. A., Mishkin, M. & Averbeck, B. B. J. Neurosci. 34, 4665–4676 (2014).
Maris, E. & Oostenveld, R. J. Neurosci. Methods 164, 177–190 (2007).
Benjamini, Y. & Hochberg, Y. J. R. Stat. Soc. B 57, 289–300 (1995).
Acknowledgements
We thank T. Sheehan, M. Trotta, A Vaz, R. Haque, A. Yang, S. Damera, B. Zavala, R. Ellenbogen, and T. Bonnevie for assistance with data collection. We thank B. Averbeck, D. Leopold, A. Martin, B. Zavala, and T. Bonnevie for helpful discussions. We thank R. Franklin and N. Halper for their technical support with collecting unit data using the Cereplex I from Blackrock Microsystems, Inc. This work was supported by the Intramural Research Program of the National Institute for Neurological Disorders and Stroke. We are indebted to all participants who have selflessly volunteered their time to participate in this study.
Author information
Authors and Affiliations
Contributions
J.H.W. and K.A.Z. conceptualized the study and wrote the paper. J.H.W. analyzed the data. J.H.W., A.I.J., and J.B.C. collected behavioral data and processed the single-unit data. J.B.C. localized iEEG electrodes. S.K.I. oversaw iEEG data acquisition and provided clinical assessment of iEEG waveforms and seizure focus localization. K.A.Z. performed all surgical procedures and supervised the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 Behavioral dissociation of attention and memory.
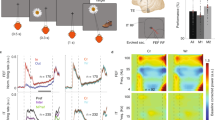
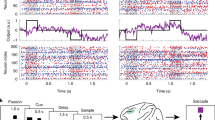
(a) Schematic of the memorization phase of the task. We instructed participants to specifically remember those words preceded by 3 rows of 12 asterisks (preparatory cue). The order of cued and uncued words was randomized. Words used in the task were randomly drawn from a pool of 3201 common nouns that were 4 to 8 characters long, and appeared sequentially and in capital letters at the center of the screen. (b) Task overview. After viewing the list of words, participants completed a series of math problems (“A + B + C = ?”) for 20 seconds to stifle sub-vocal rehearsal of the cued words and recency effects. We then tested recognition memory of the cued and uncued words using either seen/unseen or 4-alternative forced choice. Participants selected their response using keyboard arrows. Following the recognition test, we prompted participants to say all remembered “asterisk words” aloud while their responses were recorded with the laptop’s microphone. This recall test reinforced our instruction to specifically memorize the cued words. For each testing session, we included enough lists to ensure that each of the three cue conditions (preparatory, late, and uncued) had more than 20 recognition tests. (c) Task difficulty was adjusted to achieve two recognition memory performance criteria. Bars indicate mean, points are for each participant (n = 18; only criterion sessions included). (d) Timing of cue and word presentation during the memorization phase of the task. A significant improvement in recognition memory following the preparatory cue does not necessarily mean that preparatory attention mechanisms are engaged. Instead, participants might use the 3 second delay period after the word is shown to silently rehearse, refresh, and elaborate those words that they know they will have to later recall1. We tested for post-encoding strategies by randomly interleaving control trials in which a late cue comes on after the word. Recognition memory of preparatory-cue words was consistently better than that of late-cue words across participants (paired t-test, two-tailed: t(17) = 3.33, p = 0.004), demonstrating that the preparatory cue confers additional benefits before and/or during word presentation. Thus, there are two distinct periods that might reveal electrophysiological differences between cued and uncued words: the preparation phase preceding a to-be-remembered word, and the encoding phase when the word is perceived, interpreted, and stored in memory.
Supplementary Figure 2 Brain map of preparatory effect in each participant.
Each row is an individual participant (n = 18), with their specific electrode placement projected onto a standard brain, and normalized high-frequency power values (see color bar) from each electrode spatially smoothed across the surface of that brain. Gray regions on each brain show areas with no electrode within 1.25 cm. Left column, mean power during the preparatory phase of cued words. Middle column, uncued words. Right column, the difference.
Supplementary Figure 3 Whole-brain preparatory effect.
a) Mean difference in high-frequency power for every electrode analyzed (n = 18 participants). b) Across-participant t-map showing consistent differences in power for cued versus uncued trials. Gray regions indicate brain areas with less than 3 participants contributing data. c) Significant regions, uncorrected for multiple comparisons in space. d) Significant regions after permutation-based cluster correction for multiple comparisons.
Supplementary Figure 4 Whole-brain encoding effects.
a) Mean difference in high-frequency power for every electrode analyzed (n = 18 participants). b) Across-participant t-map showing consistent differences in power for cued versus uncued trials. Gray regions indicate brain areas with less than 3 participants contributing data. c) Significant regions, uncorrected for multiple comparisons in space. d) Significant regions after permutation-based cluster correction for multiple comparisons (no significant encoding effect after correction).
Supplementary Figure 5 Anterior temporal lobe suppression is negatively correlated with frontal and posterior temporal lobe enhancement.
a) Trial-by-trial correlation between the response in the anterior temporal lobe versus the frontal attention area. Points are trials. Color indicates participants (n = 15). A linear regression was fit to each participant's data for visualization purposes. b) Distribution of Spearman's rank correlation coefficients from (a) for all participants, with results from a one-sample t-test against zero (two-sided) displayed above. c–d) Trial-by-trial correlation between anterior temporal and posterior temporal responses (n = 17 participants).
Supplementary Figure 6 Attention has no effect on noise correlations in the anterior temporal lobe.
a) attention-state classifier performance (n = 11 sessions), trained using true, de-correlated (Shuf), or randomized (Chance) data from each session. Error bars indicate mean ± s.e.m. The neurons coded attentional state significantly better than expected by chance (paired t-test, two-tailed: t(10) = 3.17, p = 0.010). However, there was no significant difference in classification performance when the data was shuffled to remove correlations among neurons (paired t-test, two-tailed: t(10) = 1.6, p = 0.14), which suggests that noise correlations do not affect this population code. b) scatter plot of noise correlation for every pair of simultaneously recorded neurons (black points, n = 3168), the session-averages (red squares, n = 10), and the grand average (teal square). Dashed line is unity. There is no significant difference between cued and uncued conditions (paired t-test, two-tailed: t(3166) = 1.7, p = 0.10). c) Noise correlation versus mean pairwise spike rate (uncued trials), binned into deciles. Error bars indicate mean +/− s.e.m. (n = 11 sessions). Red points are for cued words, gray for uncued. Noise correlations are indistinguishable between conditions irrespective of rate. d) Time series of cued-uncued noise correlations. Line and shaded region indicates mean ± s.e.m. (n = 11 sessions). A sliding window of 1 second was used for the time series.
Supplementary Figure 7 Memory impairment following anterior temporal lobe resection was indistinguishable for language dominant vs nondominate resections.
Comparison of recognition accuracy changes before and after surgery for 18 participants who satisfied our performance criteria in a pre-operative testing session and subsequently had their anterior temporal lobe removed (7 language-dominant, 6 non-dominant) or completed the iEEG monitoring period and then had no tissue removed (5 no resection controls; see legend for colors associated with each group). Error bars are mean ± s.e.m. for each group (see inset for n). Top) Recognition accuracy (D-Prime) for cued and uncued words. All participants are combined for the pre-operative bar. Bottom) Within-participant differences in recognition accuracy (post-operative pre-operative). Number above each group are P-values from a paired t-test against zero (two-sided). No groups showed a significant change in recognition accuracy. However, for cued words, there was trend for more impairment with either resection compared to no resection. For the uncued words no such trend was evident.
Supplementary Figure 8 List length was varied across participants achieve our isoperformance criterion, but this task parameter did not affect the degree of anterior temporal lobe suppression.
We only included data from participants who completed at least two testing sessions satisfying our criteria (range: 2 to 9 criterion sessions per participants). Sessions that violated either performance criteria were discarded from all subsequent analyses (71 of 90 test sessions satisfied both criteria). We found that 10% of the criterion sessions also showed a significant difference between preparatory and late cued words (7 of 71 criterion sessions), with preparatory-cue performance greater than late-cue performance in all cases. For those sessions, we performed separate tests for preparatory-cue versus uncued and late-cue versus uncued and discarded two sessions in which one of those additional tests failed, leaving 69 criterion sessions across 18 participants.Adjusting task parameters most often meant adjusting list length (range: 1 to 20 cued words per list). Longer lists usually caused a bigger performance drop for uncued words than for cued words. If adjusting list length did not yield target performance, we adjusted other task parameters based on the criterion that was not met. If recognition of cued and uncued words was indistinguishable, we increased the proportion of uncued words in each list. The standard proportion was 1:1:2 (preparatory: late: uncued) words per list, but we increased this to 1:1:3 or 1:1:4 for at least 1 criterion session in 6 of 18 participants (30 of 69 total sessions). If false alarm rates were greater than 40%, we tested recognition memory using 4-alternative forced choice instead of seen/unseen (6 of 18 participants had at least one criterion 4-alternative forced choice session during the monitoring period; 28 total sessions). If recognition of cued words was less than 75% with just one or two cued words per list, we reduced the minimum required arithmetic from 20 seconds to 10 (6 sessions, 2 participants) or 5 (6 sessions, 1 participant) seconds. We note that short lists and a brief distractor period could mean that these participants were more likely to rely on working memory when solving the task, and we cannot rule this out. (a-b) distribution of list-lengths used in sessions that met our behavioral criterion. For each participant, the average length across all significant sessions is reported. (a) total list length. (b), number of preparatory + late cue words per list. (c-d) One potential concern with the variation in list lengths used across participants is that different list lengths cause different physiological effects across the participants. We confirmed that this was not the case; there was no correlation between the average difference between cued and uncued high-frequency power in the anterior temporal lobe (−0.5 to 0.0 seconds prior to word presentation) and list lengths (n = 17 participants, Spearman's rank correlation).
Supplementary Figure 9 Test for possible confound of saccadic eye movements affecting baseline period in the anterior temporal lobe.
While non-neural physiological contaminants are usually not a significant concern in intracranial human recordings, the anterior temporal lobe is especially susceptible to saccadic spike potential artifacts (Jerbi et al. Brain Topography 22, 18–23, 2009; Kovach et al. Meuroimage 54, 213–233, 2011). Even very small (<2 deg) saccades can result in significant contamination in the temporal pole up to well above 200 Hz, possibly in the spiking range. We did not track eye movements, and thus cannot guarantee that this potential noise source was eliminated using the iterative noise-removal procedure described in the Online Methods. The primary result reported in the main text is an attention-related decrease in high-frequency power and single unit spike rate in the anterior temporal lobe, which could potentially reflect the lack of eye movements on cued trials relative to our baseline period following the orientation cue. To test for this possibility, we examined the time course of high-frequency power and spiking activity surrounding the time of the orientation cue. (Top) Across-participant high-frequency power in iEEG electrodes covering the anterior temporal lobe, with all trials time-locked to the onset of the orientation cue. Lines and shaded region indicate mean ± s.e.m. of participants (n = 17). Inset shows the results of a paired t-test (two-sided) for the 500 ms before and 500 ms after the orientation cue was on the screen. (Bottom) the same analysis, but across the population of 197 isolated units. Lines and shaded region indicate mean ± s.e.m. of units (n = 197). Paired t-test is two-sided. There was no significant change in power or spike rate before versus after the orientation cue, thus, this potential confound cannot explain the observed attention-related suppression in the anterior temporal lobe.
Supplementary Figure 10 Location of iEEG electrodes used in time-series analysis (Fig. 1f–h).
A Talairach daemon was used to identified the lobe and Brodmann area associated with each electrode’s location in standard space. The anterior temporal lobe region (cyan) was selected to match the significant region in Fig. 1d. The posterior temporal lobe region (green) included all other electrodes covering the temporal lobe. The frontal attention area (black) includes all electrodes in Brodmann areas 6,8,9,44,45,4626,27 (Ptak, R. The Neuroscientist 18, 502–515, 2012; Corbetta, M. & Shulman, G.L. Nature Reviews Neuroscience 3,201–15, 2002). Electrodes that were used in the whole-brain analysis but were not in any of these three regions are shown in gray.
Supplementary Figure 11 Microelectrode array placement and single-unit isolation quality metrics.
a) Intra-operative photo showing placement of a micro-electrode array in the middle temporal gyrus (see arrow). b) CT image highlighting the subcutaneous digitizer that multiplexes 96-physiological signals onto a single wire that comes through the skin, thus minimizing infection risk. c) Distribution of Iso Scores [19] (a metric of isolation quality that ranges from 0 to 1.0, with 1 being perfectly isolated), (d) baseline firing rates, and (e) minimum trials/per condition. We found that units with isolation scores above 0.8 agreed with our strict, albeit qualitative, assessment of well-isolated units. Half of the units had mean firing rates less than 1.2 spikes/second. We eliminated all units with an average rate below one spike per encoding epoch, or 0.2 sp/s. And we eliminated any unit that was recorded for less than 18 trials per condition. (f) The mean spike waveform for all 197 units used in the analysis.
Supplementary Figure 12 Confirmation that eliminating potentially duplicate units has little effect on the main results.
Because we included multiple recording sessions for each participant, it is possible that an individual unit could contribute more than once to the population analysis, thus violating the requirement for independent samples in statistical testing. We eliminated any potential duplicate recordings, while maximizing the total number of analyzed units. We first identified whether the same physical electrode recorded units on more than one session. If so, for that electrode we kept the session with the highest number of well-isolated units. If more than one session tied for having the highest number of units, we randomly selected which session to use for that electrode as a tie breaker, leaving us with 197 independent units. We confirmed that this random selection did not affect the main findings reported here by computing the population average spike-rates including all 302 neurons that satisfied our isolation, rate, and trial count criteria, including potential duplicate recordings. (Top), populate average spike rate. (Bottom) Population average Fano factor. Lines and shaded regions indicate mean +/− s.e.m. of 302 units. Red traces indicate the cued condition, gray indicate uncued. The population response is essentially unchanged relative to the main result in Fig. 2c-d.
Supplementary information
Rights and permissions
About this article
Cite this article
Wittig, J.H., Jang, A.I., Cocjin, J.B. et al. Attention improves memory by suppressing spiking-neuron activity in the human anterior temporal lobe. Nat Neurosci 21, 808–810 (2018). https://doi.org/10.1038/s41593-018-0148-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-018-0148-7
This article is cited by
-
Backbone spiking sequence as a basis for preplay, replay, and default states in human cortex
Nature Communications (2023)
-
Micro-scale functional modules in the human temporal lobe
Nature Communications (2022)
-
Memorability of words in arbitrary verbal associations modulates memory retrieval in the anterior temporal lobe
Nature Human Behaviour (2020)
-
Feedforward prediction error signals during episodic memory retrieval
Nature Communications (2020)
-
Dynamic changes of timing precision in timed actions during a behavioural task in guinea pigs
Scientific Reports (2020)