Abstract
An approaching predator and self-motion toward an object can generate similar looming patterns on the retina, but these situations demand different rapid responses. How central circuits flexibly process visual cues to activate appropriate, fast motor pathways remains unclear. Here we identify two descending neuron (DN) types that control landing and contribute to visuomotor flexibility in Drosophila. For each, silencing impairs visually evoked landing, activation drives landing, and spike rate determines leg extension amplitude. Critically, visual responses of both DNs are severely attenuated during non-flight periods, effectively decoupling visual stimuli from the landing motor pathway when landing is inappropriate. The flight-dependence mechanism differs between DN types. Octopamine exposure mimics flight effects in one, whereas the other probably receives neuronal feedback from flight motor circuits. Thus, this sensorimotor flexibility arises from distinct mechanisms for gating action-specific descending pathways, such that sensory and motor networks are coupled or decoupled according to the behavioral state.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data and analysis code that support the findings of this study are available from the corresponding author upon reasonable request. The leg tracking software was deposited to https://github.com/kristinbranson/APT.
References
Wu, M. et al. Visual projection neurons in the Drosophila lobula link feature detection to distinct behavioral programs. eLife 5, e21022 (2016).
Panser, K. et al. Automatic segmentation of Drosophila neural compartments using GAL4 expression data reveals novel visual pathways. Curr. Biol. 26, 1943–1954 (2016).
Klapoetke, N. C. et al. Ultra-selective looming detection from radial motion opponency. Nature 551, 237–241 (2017).
von Reyn, C. R. et al. Feature integration drives probabilistic behavior in the Drosophila escape response. Neuron 94, 1190–1204 (2017).
Ache, J. M. et al. Neural basis for looming size and velocity encoding in the Drosophila giant fiber escape pathway. Curr. Biol. 29, 1073–1081.e4 (2019).
von Reyn, C. R. et al. A spike-timing mechanism for action selection. Nat. Neurosci. 17, 962–970 (2014).
Tammero, L. F. & Dickinson, M. H. Collision-avoidance and landing responses are mediated by separate pathways in the fruit fly, Drosophila melanogaster. J. Exp. Biol. 205, 2785–2798 (2002).
van Breugel, F. & Dickinson, M. H. The visual control of landing and obstacle avoidance in the fruit fly Drosophila melanogaster. J. Exp. Biol. 215, 1783–1798 (2012).
Branson, K., Robie, A. A., Bender, J., Perona, P. & Dickinson, M. H. High-throughput ethomics in large groups of Drosophila. Nat. Methods 6, 451–457 (2009).
Kim, L. H. et al. Integration of descending command systems for the generation of context-specific locomotor behaviors. Front. Neurosci. 11, 581 (2017).
Muijres, F. T., Elzinga, M. J., Melis, J. M. & Dickinson, M. H. Flies evade looming targets by executing rapid visually directed banked turns. Science 344, 172–177 (2014).
Yilmaz, M. & Meister, M. Rapid Innate defensive responses of mice to looming visual stimuli. Curr. Biol. 23, 2011–2015 (2013).
Card, G. & Dickinson, M. H. Visually mediated motor planning in the escape response of Drosophila. Curr. Biol. 18, 1300–1307 (2008).
Borst, A. Time course of the houseflies’ landing response. Biol. Cybern. 54, 379–383 (1986).
Borst, A. & Bahde, S. What kind of movement detector is triggering the landing response of the housefly? Biol. Cybern. 55, 65–69 (1986).
Bacon, J. P. & Strausfeld, N. J. The dipteran ‘Giant fibre’ pathway: neurons and signals. J. Comp. Physiol. A 158, 529–548 (1986).
Namiki, S., Dickinson, M. H., Wong, A. M., Korff, W. & Card, G. M. The functional organization of descending sensory-motor pathways in Drosophila. eLife 7, e34272 (2018).
Goodman, L. J. The landing responses of insects. J. Exp. Biol. 37, 854–878 (1960).
Baines, R. A., Uhler, J. P., Thompson, A., Sweeney, S. T. & Bate, M. Altered electrical properties in Drosophila neurons developing without synaptic transmission. J. Neurosci. 21, 1523–1531 (2001).
Borst, A. & Bahde, S. Visual information processing in the fly’s landing system. J. Comp. Physiol. A 163, 167–173 (1988).
May, M. L. & Hoy, R. R. Leg–induced steering in flying crickets. J. Exp. Biol. 151, 485–488 (1990).
Nolen, T. G. & Hoy, R. R. Initiation of behavior by single neurons: the role of behavioral context. Science 226, 992–994 (1984).
Huston, S. J. & Krapp, H. G. Nonlinear integration of visual and haltere inputs in fly neck motor neurons. J. Neurosci. 29, 13097–13105 (2009).
Bacon, J. P., Thompson, K. S. & Stern, M. Identified octopaminergic neurons provide an arousal mechanism in the locust brain. J. Neurophysiol. 74, 2739–2743 (1995).
Suver, M. P., Mamiya, A. & Dickinson, M. H. Octopamine neurons mediate flight-induced modulation of visual processing in Drosophila. Curr. Biol. 22, 2294–2302 (2012).
Tuthill, J. C., Nern, A., Rubin, G. M. & Reiser, M. B. Wide-field feedback neurons dynamically tune early visual processing. Neuron 82, 887–895 (2014).
Riemensperger, T. et al. Behavioral consequences of dopamine deficiency in the Drosophila central nervous system. Proc. Natl Acad. Sci. USA 108, 834–839 (2011).
Mamiya, A., Straw, A. D., Tomasson, E. & Dickinson, M. H. Active and passive antennal movements during visually guided steering in flying Drosophila. J. Neurosci. 31, 6900–6914 (2011).
Ramirez, J. M. Interneurons in the suboesophageal ganglion of the locust associated with flight initiation. J. Comp. Physiol. A 162, 669–685 (1988).
Maimon, G. Modulation of visual physiology by behavioral state in monkeys, mice, and flies. Curr. Opin. Neurobiol. 21, 559–564 (2011).
Suver, M. P., Huda, A., Iwasaki, N., Safarik, S. & Dickinson, M. H. An array of descending visual interneurons encoding self-motion in Drosophila. J. Neurosci. 36, 11768–11780 (2016).
Zorovic, M. & Hedwig, B. Processing of species-specific auditory patterns in the cricket brain by ascending, local, and descending neurons during standing and walking. J. Neurophysiol. 105, 2181–2194 (2011).
Hoy, R., Nolen, T. & Brodfuehrer, P. The neuroethology of acoustic startle and escape in flying insects. J. Exp. Biol. 146, 287–306 (1989).
Staudacher, E. & Schildberger, K. Gating of sensory responses of descending brain neurones during walking in crickets. J. Exp. Biol. 201, 559–572 (1998).
Haag, J., Wertz, A. & Borst, A. Central gating of fly optomotor response. Proc. Natl Acad. Sci. USA 107, 20104–20109 (2010).
Kozlov, A. K., Kardamakis, A. A., Hellgren Kotaleski, J. & Grillner, S. Gating of steering signals through phasic modulation of reticulospinal neurons during locomotion. Proc. Natl Acad. Sci. USA 111, 3591–3596 (2014).
Grillner, S., Hellgren, J., Ménard, A., Saitoh, K. & Wikström, M. A. Mechanisms for selection of basic motor programs—roles for the striatum and pallidum. Trends Neurosci. 28, 364–370 (2005).
Maimon, G., Straw, A. D. & Dickinson, M. H. Active flight increases the gain of visual motion processing in Drosophila. Nat. Neurosci. 13, 393–399 (2010).
Chiappe, M. E., Seelig, J. D., Reiser, M. B. & Jayaraman, V. Walking modulates speed sensitivity in Drosophila motion vision. Curr. Biol. 20, 1470–1475 (2010).
Coen, P., Xie, M., Clemens, J. & Murthy, M. Sensorimotor transformations underlying variability in song intensity during Drosophila courtship. Neuron 89, 629–644 (2016).
Ribeiro, I. M. A. et al. Visual projection neurons mediating directed courtship in Drosophila. Cell 174, 607–621.e18 (2018).
Fotowat, H., Fayyazuddin, A., Bellen, H. J. & Gabbiani, F. A novel neuronal pathway for visually guided escape in Drosophila melanogaster. J. Neurophysiol. 102, 875–885 (2009).
Sanes, J. R. & Zipursky, S. L. Design principles of insect and vertebrate visual systems. Neuron 66, 15–36 (2010).
Tovote, P. et al. Midbrain circuits for defensive behaviour. Nature 534, 206–212 (2016).
Pfeiffer, B. D., Truman, J. W. & Rubin, G. M. Using translational enhancers to increase transgene expression in Drosophila. Proc. Natl Acad. Sci. USA 109, 6626–6631 (2012).
Klapoetke, N. C. et al. Independent optical excitation of distinct neural populations. Nat. Methods 11, 338–346 (2014).
Tuthill, J. C., Nern, A., Holtz, S. L., Rubin, G. M. & Reiser, M. B. Contributions of the 12 neuron classes in the fly lamina to motion vision. Neuron 79, 128–140 (2013).
Pfeiffer, B. D. et al. Refinement of tools for targeted gene expression in Drosophila. Genetics 186, 735–755 (2010).
Nern, A., Pfeiffer, B. D. & Rubin, G. M. Optimized tools for multicolor stochastic labeling reveal diverse stereotyped cell arrangements in the fly visual system. Proc. Natl Acad. Sci. USA 112, E2967–E2976 (2015).
Gouwens, N. W. & Wilson, R. I. Signal propagation in Drosophila central neurons. J. Neurosci. 29, 6239–6249 (2009).
Lindsay, T., Sustar, A. & Dickinson, M. The function and organization of the motor mystem controlling flight maneuvers in flies. Curr. Biol. 27, 345–358 (2017).
Reiser, M. B. & Dickinson, M. H. A modular display system for insect behavioral neuroscience. J. Neurosci. Methods 167, 127–139 (2008).
Williamson, W. R., Peek, M. Y., Breads, P., Coop, B. & Card, G. M. Tools for rapid high-resolution behavioral phenotyping of automatically isolated Drosophila. Cell Rep. 25, 1636–1649.e5 (2018).
Brainard, D. H. The psychophysics toolbox. Spat. Vis. 10, 433–436 (1997).
Gabbiani, F., Krapp, H. G. & Laurent, G. Computation of object approach by a wide-field, motion-sensitive neuron. J. Neurosci. 19, 1122–1141 (1999).
Ian, O. A multifunctional role for octopamine in locust flight. Annu. Rev. Entomol. 38, 227–250 (1993).
Burgos-Artizzu, X. P., Perona, P. & Dollar, P. Robust face landmark estimation under occlusion. In Proc. IEEE International Conference on Computer Vision 1513–1520 (IEEE, 2013).
Ozuysal, M., Calonder, M., Lepetit, V. & Fua, P. Fast keypoint recognition using random ferns. IEEE Trans. Pattern Anal. Mach. Intell. 32, 448–461 (2010).
Acknowledgements
We thank K. von Reyn, M. Peek and S. Huston for sharing their rigs and providing software; M. Dickinson and T. Lindsay for building the Kinefly setup; M. Sumathipala, D. Parikh and P. Breads for labeling fly videos; and W. Korff and the Janelia Descending Interneuron Project for help with the DN split-GAL4 screen. We are grateful to G. Zheng and the Janelia Fly Core for fly husbandry, and to E. Gruntman and M. Reiser for sharing LED-arena code and advice. V. Jayaraman, K. Longden, E. Gruntman, C. Dallmann and Card laboratory members provided helpful comments on the manuscript. This study was supported by the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
J.M.A. conceived the project, designed experiments, conducted patch-clamp recordings, conducted behavioral and optogenetic activation experiments, wrote analysis code, analyzed the data, generated the figures and wrote the manuscript. S.N. designed experiments, conducted and analyzed behavioral optogenetic activation experiments, generated split-GAL4 driver lines and contributed anatomy figures. A.L. and K.B. developed tracking software. G.M.C. conceived the project, designed experiments, wrote the manuscript and provided supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Journal peer review information: Nature Neuroscience thanks Rachel Wilson and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
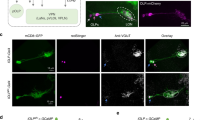
Supplementary Figure 1 DNp07 and DNp10 morphology and split-Gal4 line expression patterns.
(a) Central nervous system expression driven by the DNp10-split-GAL4 line (SS01608) was limited to only the left and right copies of DNp10. Insets in a and b show optical slices through the optic glomerulus region of the brain, indicated by gray boxes. Both DNs have dendrites close to optic glomeruli of the LPLC3/4 types. Scale bars, 50 µm. (b) Central nervous system expression pattern for DNp07-split-GAL4-2 (SS01549), used in activation and silencing experiments, which expressed strongly in DNp07, and had weaker expression in a second DN (DNp13). (c) Central nervous system expression pattern for DNp07-split-GAL4-1 (SS02276), used in activation and silencing experiments, which had off-target expression in the abdominal ganglia, but did not express in other DNs. Panels a, b, c, CsChrimson-mVenus expression; panels d, e, and insets in a, b, GFP expression. (d) Unilateral MultiColor FlpOut of DNp10, DNp07, and DNp13. (e) Saggital view of DNp10, DNp07, and DNp13. Note that DNp13 does not innervate the leg neuropil areas (dashed circles). For all panels, similar results were obtained from N = 4 different flies.
Supplementary Figure 2 Movements elicited by DNp07 and DNp10 activation resemble visually evoked landing responses.
(a) Video frames from a visually evoked landing response in a tethered fly (inset in c), captured at 1000 Hz. Stimulus was a unilateral bar moving front to back at 1000 °/s. Cyan line and circle in right panel indicates body axis orientation for c-e. (b) Still image of a free flying fly landing spontaneously on the ground, rotated to match the body orientation of tethered flies. (c) Example leg tip trajectories from four different flies viewing the same stimulus as in a. Leg tip positions for ipsilateral middle and hind legs and both front legs are shown every 5 ms for 200 frames. Dashed arrows (Fly 1) indicate the vector length used to quantify the leg extension amplitude in the graphs on right and main Fig. 2. Gray shading indicates visual stimulus. All legs moved in-phase with each other. Hind leg movements were more variable than front and middle leg movements. (d) Example leg tip trajectories elicited by DNp07-split-GAL4-1 activation (100% intensity), shown every 5 ms for 50 frames. Orange shading indicates optogenetic stimulus. (e) Same as d for DNp10 activation (75% intensity). Leg trajectories and body attitude were rotated so that all body axes are oriented horizontally.
Supplementary Figure 3 DNp07 and DNp10 drive landing responses with different middle and hind leg postures upon optogenetic activation.
(a) Optogenetic activation of DNp07 (DNp07-split-GAL4-2, magenta) or DNp10 (blue) extends front, middle, and hind legs, shown as leg tip position change from start to peak during a 300-ms light pulse. Light colors show fly means, dark arrows population average. BL, body length. (b) Boxplots show median and quartiles of leg extension angles during DNp07 and DNp10 activation relative to initial position. N = 6 flies, N = 12 legs, n = 4-5 trials each. Data from left legs was reflected and included. ***P = 5.9x10-4, *P = 0.0304 (two-sided Wilcoxon rank sum test). Note that the two outliers (black) in b have minute amplitudes in a.
Supplementary Figure 4 DNp07 and DNp10 silencing experiments.
(a) Tethered flies were shown visual stimuli, including a 500°/s fast, 10°-wide, dark, front-to back moving bar (the same stimulus we used for electrophysiological experiments), frontal looming (r/v = 80), and bilateral frontal expansion (500°/s). (b) Still video frames indicate ROI (orange circles) used for automatic detection of front leg extension during the landing response. (c) Example trace of normalized mean pixel intensity of the front leg ROI, smoothed over a 50-ms time window. Sequence shows leg movement responses (orange) to three subsequent visual stimuli (stimulus time courses in gray). ROI pixel intensity traces were used to calculate time points of landing responses by applying a constant threshold (3x SD) for each fly (dashed line). (d) Takeoff was not impaired in DNp07-split-GAL4-1 or DNp10-silenced flies. Bars, show mean + SD takeoff fraction; dots, individual fly means. NGFP = 9, NDNp07 = 11, NDNp10 = 10 different flies. Flight starts in this case were without ground contact. (e) Examples of ROI intensity traces from N = 3 flies each for three genotypes showing landing responses (black or colored) or no landing responses (gray) to n = 18 presentations of looming stimuli (below). For each genotype, an example of a fly with a low, intermediate, and high response rate is shown. Dotted lines indicate an approximation of the onset time of ROI intensity changes, when the leg moved into the ROI, for GFP controls. DNp07 examples are using the DNp07-split-GAL4-1 line.
Supplementary Figure 5 DNp07 and DNp10 have similar visual tuning. We visually stimulated the right eye while recording the ipsi- (right) or contralateral (left) copy of each DN.
(a-c) DN responses (mean ± SD, sample sizes defined in b; N, number of recordings from different flies) to different speeds of front-to-back motion of bars (a), small squares (b), or lateral looming stimuli presented at 45° azimuth, and 45° elevation (c) (which, unlike frontal looming stimuli, do not strongly drive landing responses). (d) Directional tuning of landing DNs, dots show the mean ± SD peak firing rate in 20-ms bins during stimulation with 100°/s fast moving bars. N = 6 ipsilateral, N = 4 contralateral recordings per DN. Inset shows directions of motion, u, up, d, down, b, back-to-front, f, front-to-back. (e) Both DNs responded more strongly to dark compared to bright objects. The contrast selectivity was calculated from stimulation with bright, b, and dark, d, edges moving front-to-back at 500°/s (example responses from DNp07). +1 indicates response to dark edges only, -1 bright edges only. N = 11 DNp10, N = 4 DNp07 recordings from different flies. Panels a-e all from flight trials only. (f) DNp07 recordings from a setup with no leg tracking, permitting bilateral visual stimulation with dark-edge front-to-back motion (insets), providing an expansion cue that mimics frontal object approach. Right, mean number of spikes from bilateral stimulation was comparable to the linear sum of unilateral responses. All responses in f from non-flight trials. N, number of recordings from different flies; n, number of trials per fly.
Supplementary Figure 6 Timing and magnitude of leg movements during flight were correlated with landing DN activity.
(a) DNp07 spike times, example intracellular recording, spike rate, and middle leg extension amplitude vector for five examples of visually-evoked landing responses (see Supplementary Figure 2c) aligned to the timing of the first spike. One non-spiking trial (lightest gray) was aligned to peak leg extension. (b) Same plot details as in a, data from DNp10. (c) Additional example time traces of the DNp10 spike rate (in 100-ms bins) and the middle leg extension vector during different visual stimuli. (d) A data set of 41 trials from the same fly during one DNp07 recording was separated and pooled into three groups according to peak leg extension amplitude: 0-30th percentile (left), 30-70th percentile (middle), and >70th percentile (right). Traces show mean leg extension vector (black) and spike rates (magenta) aligned to movement onset. (e) Plot details as in d, for 39 trials during one DNp10 recording.
Supplementary Figure 7 Optogenetic control of landing DN spike rate.
(a-b) Whole cell DNp07 and DNp10 recordings during 50-ms optogenetic stimulation (orange) at different light intensities, indicated as percent of LED driver power. Spike time rasters (black) are shown for five subsequent trials at each intensity. Inset on lower left shows the first five spikes for three subsequent trials of DNp07 activation. Automatic spike detection was accurate, even during large depolarizations resulting in high frequency spiking. (c-d) DN spike rate depended linearly on LED intensity. Gray lines, spike rate mean ± SD (shading) per fly; colored lines, linear fits to population data. N, number of recordings from different flies. (e) Light intensities at different LED power settings, measured at the position of the fly’s head.
Supplementary Figure 8 Dopamine does not mediate the flight-dependent increase of DNp10 visual responses.
(a) Mean ± SD spike rate of DNp10 during presentation of front-to-back moving bars of different velocities in non-flying flies. N, number of recordings from different flies. (b) Visual responses of the same flies to the same set of stimuli after application of 10 mM DA. N, number of recordings from different flies. (c) Example responses to 500°/s fast bar during non-flight (black) non-flight + DA (orange), and flight after DA application (gray). All trials from the same fly.
Supplementary Figure 9 Landing DNs are multimodal and respond to mechanosensory stimulation via air puffs.
(a). Spike rate (upper traces) and membrane potential of DNp10 during 1-s air puff stimulation (cyan bar). Different colors indicate different manipulations. Ablation of the ipsi- (-i. ant, dark orange) or contralateral (-c. ant, light orange) antenna halved the depolarization of DNp10, and ablation of both antennae eliminated spiking in DNp10. In contrast, DNp07 was inhibited by air puffs. Ablation of both antennae (no ant., black.) reduced the hyperpolarization of DNp07 strongly and additional ablation of the legs (no ant. and no legs, gray) nearly eliminated the mechanosensory response. All traces in a are population means. (b) Quantification of ablation experiments. Plots show trial means (small dots) and grand mean (large dots) of membrane potential calculated over a 900-ms window starting 100 ms after air puff onset for each condition. ***P = 3x10-9, t = 9.1, df = 24 (two-sided t-test). **P = 0.0017 , t = -3.6, df = 22 (two-sided t-test). Sample sizes given in a. All data from non-flying flies. Air puffs resulted in DNp10 excitation independent of whether or not they elicited a flight bout.
Supplementary Figure 10 Schematic model for state-dependent decoupling of sensory and motor networks.
In non-flying flies (left), visual responses of landing DNs (DNp07, magenta, and DNp10, blue) are gated out. The activity of sensory networks activated by visual stimuli such as looming cues can therefore not drive landing motor patterns in the VNC. Other descending pathways, like those driving takeoff (black), are not gated out and hence responsive. In this case, looming visual stimuli are channeled into the escape takeoff pathway in non-flight, and the fly takes off in response to a looming stimulus. In flight (right), landing DNs are gated in via octopaminergic neuromodulation (OA, orange) or by intrinsic feedback from flight motor circuits (FB, green), such that visual responses drive landing DNs and, as a result, landing motor patterns in the VNC. Though not directly measured here, descending neurons for escape may be gated out during flight, since in this state visual stimuli do not evoke escape-related behaviors, such as rapid middle leg extension (see Videos M1-2 and M8-10), and instead frontal visual looming stimuli activate the landing pathway during flight.
Supplementary Figure 11 Optogenetic excitability of landing DNs is similar in flight and non-flight.
(a) Optogenetic activation of DNp07 elicited similar responses independent of whether flies were flying or not. (b) Boxplots show median and quartiles of spike numbers per trial during flight and non-flight bouts for N = 3 flies and n trials per fly and condition; dashes, outliers. On average, about one additional spike was elicited during non-flight bouts in DNp07. (c-d) Same plot details as (a-b), data from DNp10, N = 4 flies and n trials per fly and condition. The same number of spikes was elicited during optogenetic DNp10 activation, independent of whether or not the fly was flying. Dotted line in c shows hyperpolarized non-flight resting potential. P-values are from two-sided Wilcoxon Signed Rank test, sample sizes (n, number of trials per condition) are indicated above.
Supplementary information
Supplementary Video 1
Example of DNp10 > GFP control fly responses to repeated presentation of frontal visual looming stimuli. Video was captured at 100 Hz. Each trial sequence is 1.5s long and includes the last second of looming expansion and 500 ms after the stimulus stopped moving (see also Supplementary Fig. 2d). Video is slowed-down 6x. All trials were scored as landing trials
41593_2019_413_MOESM4_ESM.mp4
Supplementary Video 2 Example of DNp07 > GFP control fly responses to repeated presentation of frontal visual looming stimuli. Video was captured at 100 Hz. Each trial sequence is 1.5s long and includes the last second of looming expansion and 500 ms after the stimulus stopped moving (see also Supplementary Fig. 2d). Video is slowed-down 6x. All trials were scored as landing trials
41593_2019_413_MOESM5_ESM.mp4
Supplementary Video 3 Spontaneous landing approach in free flight. Flies landing spontaneously extend their leg to the typical landing pose. Video was captured at 600 Hz and slowed down 200x
41593_2019_413_MOESM6_ESM.mp4
Supplementary Video 4 Optogenetic activation of DNp07 reliably drives landing responses. Montage shows 20 trials of the same fly responding to 300-ms long activation of DNp07 (DNp07-split-GAL4-2 > UAS-CsChrimson) at 100% intensity. Video was captured at 125 Hz and slowed down to 1/5 real time. White square indicates optogenetic stimulation LED on
41593_2019_413_MOESM7_ESM.mp4
Supplementary Video 5 Optogenetic activation of DNp10 reliably drives landing responses. Montage shows 20 trials of the same fly responding to 300-ms long activation of DNp10 (DNp10-split-GAL4 > UAS-CsChrimson) at 100% intensity. Video was captured at 125 Hz and slowed down to 1/5 real time. White square indicates optogenetic stimulation LED on
41593_2019_413_MOESM8_ESM.avi
Supplementary Video 6 DNp07 activation drives landing responses in flight and similar leg movements in non-flight bouts. Montage shows six trials of the same fly responding to DNp07 activation at 100% intensity. The time stamp shows time relative to stimulus onset, the white dot in the top right indicates timing of Chrimson LED. The top row shows flight trials; the bottom row shows non-flight trials. The frame rate was 1, 000 Hz. Similar videos were acquired for N = 4 flies. Note that DN activation initiated flight in some trials, which had much longer latencies than leg movements. Trials with ground contact resulted in similar leg movements without flight starts (data not shown). Genotype used was DNp07-split-GAL4-1 > UAS-CsChrimson
41593_2019_413_MOESM9_ESM.avi
Supplementary Video 7 DNp10 activation drives landing responses in flight and similar leg movements in non-flight bouts. Montage of six trials of the same fly responding to DNp10 activation at 100% intensity. Details as for Supplementary Video 6. Genotype used was DNp10-split-GAL4 > UAS-CsChrimson
41593_2019_413_MOESM10_ESM.mp4
Supplementary Video 8 Example DNp07-silenced fly responses to repeated presentation of frontal visual looming stimuli. Video was captured at 100 Hz. Each trial sequence is 1.5s long and includes the last second of looming expansion and 500 ms after the stimulus stopped moving (see also Supplementary Fig. 2d). Video is slowed-down 6x. None of the trials was scored as a landing trial. Genotype used was DNp07-split-GAL4-1 > UAS-Kir2.1
41593_2019_413_MOESM11_ESM.mp4
Supplementary Video 9 Example DNp10-silenced fly responses to repeated presentation of frontal visual looming stimuli. Video was captured at 100 Hz. Each trial sequence is 1.5s long and includes the last second of looming expansion and 500 ms after the stimulus stopped moving (see also Supplementary Fig. 2d). Video is slowed-down 6x. Trial 10 was scored as a landing trial. Several other trials show partial leg extensions. Genotype used was DNp10-split-GAL4 > UAS-Kir2.1
41593_2019_413_MOESM12_ESM.mp4
Supplementary Video 10 Example DNp10-silenced fly responses to repeated presentation of frontal visual looming stimuli. Video was captured at 100 Hz. Each trial sequence is 1.5 s long and includes the last second of looming expansion and 500 ms after the stimulus stopped moving (see also Supplementary Fig. 2d). Video is slowed-down 6x. Trials 1, 3, 4, 5, 9 and 10 were scored as landing trials. Several other trials show partial leg extensions. Genotype used was DNp10-split-GAL4 > UAS-Kir2.1
Rights and permissions
About this article
Cite this article
Ache, J.M., Namiki, S., Lee, A. et al. State-dependent decoupling of sensory and motor circuits underlies behavioral flexibility in Drosophila. Nat Neurosci 22, 1132–1139 (2019). https://doi.org/10.1038/s41593-019-0413-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-019-0413-4
This article is cited by
-
Model organisms and systems in neuroethology: one hundred years of history and a look into the future
Journal of Comparative Physiology A (2024)
-
Synaptic gradients transform object location to action
Nature (2023)
-
Different spectral sensitivities of ON- and OFF-motion pathways enhance the detection of approaching color objects in Drosophila
Nature Communications (2023)
-
Flexible circuit mechanisms for context-dependent song sequencing
Nature (2023)
-
The spatial and temporal structure of neural activity across the fly brain
Nature Communications (2023)