Abstract
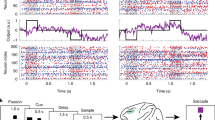
Recently it has been proposed that information in working memory (WM) may not always be stored in persistent neuronal activity but can be maintained in ‘activity-silent’ hidden states, such as synaptic efficacies endowed with short-term synaptic plasticity. To test this idea computationally, we investigated recurrent neural network models trained to perform several WM-dependent tasks, in which WM representation emerges from learning and is not a priori assumed to depend on self-sustained persistent activity. We found that short-term synaptic plasticity can support the short-term maintenance of information, provided that the memory delay period is sufficiently short. However, in tasks that require actively manipulating information, persistent activity naturally emerges from learning, and the amount of persistent activity scales with the degree of manipulation required. These results shed insight into the current debate on WM encoding and suggest that persistent activity can vary markedly between short-term memory tasks with different cognitive demands.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Data from all trained networks that were analyzed for this study are available from the corresponding author upon reasonable request.
Code availability
The code used to train, simulate and analyze network activity is available at https://github.com/nmasse/Short-term-plasticity-RNN
References
Baddeley, A. D. & Hitch, G. Working memory. Psychol. Learn. Motiv. 8, 47–89 (1974).
Funahashi, S., Bruce, C. J. & Goldman-Rakic, P. S. Mnemonic coding of visual space in the monkey’s dorsolateral prefrontal cortex. J. Neurophysiol. 6, 331–349 (1989).
Chafee, M. V. & Goldman-Rakic, P. S. Matching patterns of activity in primate prefrontal area 8a and parietal area 7ip neurons during a spatial working memory task. J. Neurophysiol. 79, 2919–2940 (1998).
Colby, C. L., Duhamel, J. R. & Goldberg, M. E. Visual, presaccadic, and cognitive activation of single neurons in monkey lateral intraparietal area. J. Neurophysiol. 76, 2841–2852 (1996).
Romo, R., Brody, C. D., Hernández, A. & Lemus, L. Neuronal correlates of parametric working memory in the prefrontal cortex. Nature 399, 470–473 (1999).
Rainer, G., Asaad, W. F. & Miller, E. K. Selective representation of relevant information by neurons in the primate prefrontal cortex. Nature 393, 577–579 (1998).
Wang, M. et al. NMDA receptors subserve persistent neuronal firing during working memory in dorsolateral prefrontal cortex. Neuron 77, 736–749 (2013).
Wang, X.-J. Synaptic basis of cortical persistent activity: the importance of NMDA receptors to working memory. J. Neurosci. 19, 9587–9603 (1999).
Floresco, S. B., Braaksma, D. N. & Phillips, A. G. Thalamic-cortical-striatal circuitry subserves working memory during delayed responding on a radial arm maze. J. Neurosci. 19, 11061–11071 (1999).
Masse, N. Y., Hodnefield, J. M. & Freedman, D. J. Mnemonic encoding and cortical organization in parietal and prefrontal cortices. J. Neurosci. 37, 6098–6112 (2017).
Stokes, M. G. ‘Activity-silent’ working memory in prefrontal cortex: a dynamic coding framework. Trends Cogn. Sci. 19, 394–405 (2015).
Lara, A. H. & Wallis, J. D. Executive control processes underlying multi-item working memory. Nat. Neurosci. 17, 876–883 (2014).
Watanabe, K. & Funahashi, S. Neural mechanisms of dual-task interference and cognitive capacity limitation in the prefrontal cortex. Nat. Neurosci. 17, 601–611 (2014).
Sreenivasan, K. K., Curtis, C. E. & D’Esposito, M. Revisiting the role of persistent neural activity during working memory. Trends Cogn. Sci. 18, 82–89 (2014).
Lee, S.-H., Kravitz, D. J. & Baker, C. I. Goal-dependent dissociation of visual and prefrontal cortices during working memory. Nat. Neurosci. 16, 997–999 (2013).
Sarma, A., Masse, N. Y., Wang, X.-J. & Freedman, D. J. Task-specific versus generalized mnemonic representations in parietal and prefrontal cortices. Nat. Neurosci. 19, 143–149 (2016).
Zucker, R. S. & Regehr, W. G. Short-term synaptic plasticity. Annu. Rev. Physiol. 64, 355–405 (2002).
Mongillo, G., Barak, O. & Tsodyks, M. Synaptic theory of working memory. Science 319, 1543–1546 (2008).
Rose, N. S. et al. Reactivation of latent working memories with transcranial magnetic stimulation. Science 354, 1136–1139 (2016).
Wolff, M. J., Jochim, J., Akyürek, E. G. & Stokes, M. G. Dynamic hidden states underlying working-memory-guided behavior. Nat. Neurosci. 20, 864–871 (2017).
Koenigs, M., Barbey, A. K., Postle, B. R. & Grafman, J. Superior parietal cortex is critical for the manipulation of information in working memory. J. Neurosci. 29, 14980–14986 (2009).
D’Esposito, M., Postle, B. R., Ballard, D. & Lease, J. Maintenance versus manipulation of information held in working memory: an event-related fMRI study. Brain Cogn. 41, 66–86 (1999).
Mante, V., Sussillo, D., Shenoy, K. V. & Newsome, W. T. Context-dependent computation by recurrent dynamics in prefrontal cortex. Nature 503, 78–84 (2013).
Song, H. F., Yang, G. R. & Wang, X.-J. Reward-based training of recurrent neural networks for cognitive and value-based tasks. eLife 6, e21492 (2017).
Chaisangmongkon, W., Swaminathan, S. K., Freedman, D. J. & Wang, X.-J. Computing by robust transience: how the fronto-parietal network performs sequential, category-based decisions. Neuron 93, 1504–1517.e4 (2017).
Wang, J., Narain, D., Hosseini, E. A. & Jazayeri, M. Flexible timing by temporal scaling of cortical responses. Nat. Neurosci. 21, 102–110 (2018).
Goudar, V. & Buonomano, D. V. Encoding sensory and motor patterns as time-invariant trajectories in recurrent neural networks. eLife 7, e31134 (2018).
Issa, E. B., Cadieu, C. F. & DiCarlo, J. J. Neural dynamics at successive stages of the ventral visual stream are consistent with hierarchical error signals. Preprint at bioRxiv https://www.biorxiv.org/content/10.1101/092551v2 (2018).
Wang, J. X. et al. Prefrontal cortex as a meta-reinforcement learning system. Nat. Neurosci. 21, 860–868 (2018).
Song, H. F., Yang, G. R. & Wang, X.-J. Training excitatory-inhibitory recurrent neural networks for cognitive tasks: a simple and flexible framework. PLoS Comput. Biol. 12, e1004792 (2016).
Vinje, W. E. & Gallant, J. L. Sparse coding and decorrelation in primary visual cortex during natural vision. Science 287, 1273–1276 (2000).
Olshausen, B. & FIELD, D. Sparse coding of sensory inputs. Curr. Opin. Neurobiol. 14, 481–487 (2004).
Laughlin, S. B., de Ruyter van Steveninck, R. R. & Anderson, J. C. The metabolic cost of neural information. Nat. Neurosci. 1, 36–41 (1998).
Rainer, G., Rao, S. C. & Miller, E. K. Prospective coding for objects in primate prefrontal cortex. J. Neurosci. 19, 5493–5505 (1999).
Weissman, D. H., Roberts, K. C., Visscher, K. M. & Woldorff, M. G. The neural bases of momentary lapses in attention. Nat. Neurosci. 9, 971–978 (2006).
Miller, E. K., Erickson, C. A. & Desimone, R. Neural mechanisms of visual working memory in prefrontal cortex of the macaque. J. Neurosci. 16, 5154–5167 (1996).
Schneegans, S. & Bays, P. M. Restoration of fMRI decodability does not imply latent working memory States. J. Cogn. Neurosci. 29, 1977–1994 (2017).
Mendoza-Halliday, D., Torres, S. & Martinez-Trujillo, J. C. Sharp emergence of feature-selective sustained activity along the dorsal visual pathway. Nat. Neurosci. 17, 1255–1262 (2014).
Kornblith, S., Quian Quiroga, R., Koch, C., Fried, I. & Mormann, F. Persistent single-neuron activity during working memory in the human medial temporal lobe. Curr. Biol. 27, 1026–1032 (2017).
Takeda, K. & Funahashi, S. Population vector analysis of primate prefrontal activity during spatial working memory. Cereb. Cortex 14, 1328–1339 (2004).
Buschman, T. J., Siegel, M., Roy, J. E. & Miller, E. K. Neural substrates of cognitive capacity limitations. Proc. Natl Acad. Sci. USA 108, 11252–11255 (2011).
Trübutschek, D., Marti, S., Ueberschär, H. & Dehaene, S. Probing the limits of activity-silent non-conscious working memory. Preprint at bioRxiv https://www.biorxiv.org/content/10.1101/379537v1 (2018).
Orhan, A. E. & Ma, W. J. A diverse range of factors affect the nature of neural representations underlying short-term memory. Nat. Neurosci. 22, 275–283 (2019).
Wang, X. J. Synaptic reverberation underlying mnemonic persistent activity. Trends Neurosci. 24, 455–463 (2001).
Salazar, R. F., Dotson, N. M., Bressler, S. L. & Gray, C. M. Content-specific fronto-parietal synchronization during visual working memory. Science 338, 1097–1100 (2012).
Lundqvist, M. et al. Gamma and beta bursts underlie working memory. Neuron 90, 152–164 (2016).
Bolkan, S. S. et al. Thalamic projections sustain prefrontal activity during working memory maintenance. Nat. Neurosci. 20, 987–996 (2017).
Hochreiter, S. & Schmidhuber, J. Long short-term memory. Neural Comput. 9, 1735–1780 (1997).
Yang, G. R., Joglekar, M. R., Song, H. F., Newsome, W. T. & Wang, X.-J. Task representations in neural networks trained to perform many cognitive tasks. Nat. Neurosci. 22, 297–306 (2019).
Abadi, M. et al. TensorFlow: large-scale machine learning on heterogeneous distributed systems. Preprint at arXiv https://arxiv.org/abs/1603.04467 (2016).
Kingma, D. P. & Ba, J. Adam: a method for stochastic optimization. Preprint at arXiv https://arxiv.org/abs/1412.6980 (2014).
Swaminathan, S. K. & Freedman, D. J. Preferential encoding of visual categories in parietal cortex compared with prefrontal cortex. Nat. Neurosci. 15, 315–320 (2012).
Acknowledgements
This work was supported by National Institutes of Health grants R01EY019041 and R01MH092927, National Science Foundation Career Award NCS 1631571 and Department of Defense VBFF.
Author information
Authors and Affiliations
Contributions
N.Y.M, G.R.Y., H.F.S., X.J.W. and D.J.F. contributed to conceiving the research. N.Y.M. performed all model simulations and data analysis. N.Y.M and D.J.F wrote the manuscript, which was further edited by G.R.Y., H.F.S. and X.J.W.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Journal peer review information: Nature Neuroscience thanks Timothy Buschman, Michael Frank, Daniel Scott and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Masse, N.Y., Yang, G.R., Song, H.F. et al. Circuit mechanisms for the maintenance and manipulation of information in working memory. Nat Neurosci 22, 1159–1167 (2019). https://doi.org/10.1038/s41593-019-0414-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-019-0414-3
This article is cited by
-
Causation in neuroscience: keeping mechanism meaningful
Nature Reviews Neuroscience (2024)
-
A distributed and efficient population code of mixed selectivity neurons for flexible navigation decisions
Nature Communications (2023)
-
Low-dimensional encoding of decisions in parietal cortex reflects long-term training history
Nature Communications (2023)
-
Multiplexing working memory and time in the trajectories of neural networks
Nature Human Behaviour (2023)
-
Neural Mechanisms of the Maintenance and Manipulation of Gustatory Working Memory in Orbitofrontal Cortex
Cognitive Computation (2023)