Abstract
Unlike the sensory thalamus, studies on the functional organization of the midline and intralaminar nuclei are scarce, and this has hindered the establishment of conceptual models of the function of this brain region. We investigated the functional organization of the paraventricular nucleus of the thalamus (PVT), a midline thalamic structure that is increasingly being recognized as a critical node in the control of diverse processes such as arousal, stress, emotional memory and motivation, in mice. We identify two major classes of PVT neurons—termed type I and type II—that differ in terms of gene expression, anatomy and function. In addition, we demonstrate that type II neurons belong to a previously neglected class of PVT neurons that convey arousal-related information to corticothalamic neurons of the infralimbic cortex. Our results uncover the existence of an arousal-modulated thalamo-corticothalamic loop that links the PVT and the ventromedial prefrontal cortex.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All the data that support the findings presented in this study are available from the corresponding author on reasonable request.
References
Groenewegen, H. J. & Berendse, H. W. The specificity of the ‘nonspecific’ midline and intralaminar thalamic nuclei. Trends Neurosci. 17, 52–57 (1994).
Van der Werf, Y. D., Witter, M. P. & Groenewegen, H. J. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res. Brain Res. Rev. 39, 107–140 (2002).
Matyas, F. et al. A highly collateralized thalamic cell type with arousal-predicting activity serves as a key hub for graded state transitions in the forebrain. Nat. Neurosci. 21, 1551 (2018).
Ren, S. et al. The paraventricular thalamus is a critical thalamic area for wakefulness. Science 362, 429–434 (2018).
Penzo, M. A. et al. The paraventricular thalamus controls a central amygdala fear circuit. Nature 519, 455–459 (2015).
Do-Monte, F. H., Quinones-Laracuente, K. & Quirk, G. J. A temporal shift in the circuits mediating retrieval of fear memory. Nature 519, 460–463 (2015).
Do-Monte, F. H., Minier-Toribio, A., Quinones-Laracuente, K., Medina-Colon, E. M. & Quirk, G. J. Thalamic regulation of sucrose seeking during unexpected reward omission. Neuron 94, 388–400.e384 (2017).
Kirouac, G. J. Placing the paraventricular nucleus of the thalamus within the brain circuits that control behavior. Neurosci. Biobehav. Rev. 56, 315–329 (2015).
Su, H. S. & Bentivoglio, M. Thalamic midline cell populations projecting to the nucleus accumbens, amygdala, and hippocampus in the rat. J. Comp. Neurol. 297, 582–593 (1990).
Moga, M. M., Weis, R. P. & Moore, R. Y. Efferent projections of the paraventricular thalamic nucleus in the rat. J. Comp. Neurol. 359, 221–238 (1995).
Choi, E. A., Jean-Richard-Dit-Bressel, P., Clifford, C. W. G. & McNally, G. P. Paraventricular thalamus controls behavior during motivational conflict. J. Neurosci. 39, 4945–4958 (2019).
Barson, J. R. & Leibowitz, S. F. GABA-induced inactivation of dorsal midline thalamic subregions has distinct effects on emotional behaviors. Neurosci. Lett. 609, 92–96 (2015).
Choi, D. L. et al. Orexin signaling in the paraventricular thalamic nucleus modulates mesolimbic dopamine and hedonic feeding in the rat. Neuroscience 210, 243–248 (2012).
Bhatnagar, S. et al. A cholecystokinin-mediated pathway to the paraventricular thalamus is recruited in chronically stressed rats and regulates hypothalamic-pituitary-adrenal function. J. Neurosci. 20, 5564–5573 (2000).
Hua, R. F. et al. Calretinin neurons in the midline thalamus modulate starvation-induced arousal. Curr. Biol. 28, 3948 (2018).
Zeng, H. & Sanes, J. R. Neuronal cell-type classification: challenges, opportunities and the path forward. Nat. Rev. Neurosci. 18, 530–546 (2017).
Rieck, R. W., Ansari, M. S., Whetsell, W. O. Jr., Deutch, A. Y. & Kessler, R. M. Distribution of dopamine D2-like receptors in the human thalamus: autoradiographic and PET studies. Neuropsychopharmacology 29, 362–372 (2004).
Clark, A. M. et al. Dopamine D2 receptors in the paraventricular thalamus attenuate cocaine locomotor sensitization. eNeuro https://doi.org/10.1523/ENEURO.0227-17.2017 (2017).
Beas, B. S. et al. The locus coeruleus drives disinhibition in the midline thalamus via a dopaminergic mechanism. Nat. Neurosci. 21, 963–973 (2018).
Gerfen, C. R., Paletzki, R. & Heintz, N. GENSAT BAC cre-recombinase driver lines to study the functional organization of cerebral cortical and basal ganglia circuits. Neuron 80, 1368–1383 (2013).
Qualls-Creekmore, E. et al. Galanin-Expressing GABA neurons in the lateral hypothalamus modulate food reward and noncompulsive locomotion. J. Neurosci. 37, 6053–6065 (2017).
Ago, Y. et al. The female encounter test: a novel method for evaluating reward-seeking behavior or motivation in mice. Int. J. Neuropsychopharmacol. 18, pyv062 (2015).
Tan, C. L. et al. Warm-sensitive neurons that control body temperature. Cell 167, 47–59 e15 (2016).
Llinas, R. Consciousness and the thalamocortical loop. Int. Congr. Ser. 1250, 409–416 (2003).
Briggs, F. & Usrey, W. M. Emerging views of corticothalamic function. Curr. Opin. Neurobiol. 18, 403–407 (2008).
Li, S. & Kirouac, G. J. Sources of inputs to the anterior and posterior aspects of the paraventricular nucleus of the thalamus. Brain Struct. Funct. 217, 257–273 (2012).
Hurley, K. M., Herbert, H., Moga, M. M. & Saper, C. B. Efferent projections of the infralimbic cortex of the rat. J. Comp. Neurol. 308, 249–276 (1991).
Otis, J. M. et al. Paraventricular thalamus projection neurons integrate cortical and hypothalamic signals for cue-reward processing. Neuron 103, 423–431.e4 (2019).
Wolff, M. & Vann, S. D. The cognitive thalamus as a gateway to mental representations. J. Neurosci. 39, 3–14 (2019).
Klapoetke, N. C. et al. Independent optical excitation of distinct neural populations. Nat. Methods 11, 338–346 (2014).
Murillo-Rodriguez, E., Palomero-Rivero, M., Millan-Aldaco, D. & Di Marzo, V. The administration of endocannabinoid uptake inhibitors OMDM-2 or VDM-11 promotes sleep and decreases extracellular levels of dopamine in rats. Physiol. Behav. 109, 88–95 (2013).
Santini, E., Quirk, G. J. & Porter, J. T. Fear conditioning and extinction differentially modify the intrinsic excitability of infralimbic neurons. J. Neurosci. 28, 4028–4036 (2008).
Giustino, T. F., Fitzgerald, P. J. & Maren, S. Fear expression suppresses medial prefrontal cortical firing in rats. PLoS One 11, e0165256 (2016).
Leuchs, L., Schneider, M., Czisch, M. & Spoormaker, V. I. Neural correlates of pupil dilation during human fear learning. Neuroimage 147, 186–197 (2017).
Bradley, M. M., Miccoli, L., Escrig, M. A. & Lang, P. J. The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology 45, 602–607 (2008).
Zhang, S. et al. Ventromedial prefrontal cortex and the regulation of physiological arousal. Soc. Cogn. Affect. Neur. 9, 900–908 (2014).
Paxinos, G. & Watson, C. The Rat Brain in Stereotaxic Coordinates, 7th edn. (Elsevier Academic Press, 2014).
Paxinos G. & Franklin, K. B. J. The Mouse Brain in Stereotaxic Coordinates. 2nd edn (Elsevier Academic Press, 2004).
Phillips, J. W. et al. A repeated molecular architecture across thalamic pathways. Nat. Neurosci. 22, 1925–1935 (2019).
Chung, S. et al. Identification of preoptic sleep neurons using retrograde labelling and gene profiling. Nature 545, 477–481 (2017).
Bhatnagar, S., Huber, R., Nowak, N. & Trotter, P. Lesions of the posterior paraventricular thalamus block habituation of hypothalamic-pituitary-adrenal responses to repeated restraint. J. Neuroendocrinol. 14, 403–410 (2002).
Meffre, J. et al. Orexin in the posterior paraventricular thalamus mediates hunger-related signals in the nucleus accumbens core. Curr. Biol. 29, 3298–3306.e4 (2019).
Choi, E. A. & McNally, G. P. Paraventricular thalamus balances danger and reward. J. Neurosci. 37, 3018–3029 (2017).
Lang, P. J. The emotion probe. Studies of motivation and attention. Am. Psychol 50, 372–385 (1995).
Mandelbaum, G. et al. Distinct cortical-thalamic-striatal circuits through the parafascicular nucleus. Neuron 102, 636–652.e637 (2019).
Sotres-Bayon, F. & Quirk, G. J. Prefrontal control of fear: more than just extinction. Curr. Opin. Neurobiol. 20, 231–235 (2010).
Do-Monte, F. H., Manzano-Nieves, G., Quinones-Laracuente, K., Ramos-Medina, L. & Quirk, G. J. Revisiting the role of infralimbic cortex in fear extinction with optogenetics. J. Neurosci. 35, 3607–3615 (2015).
Vanderkooy, D., Koda, L. Y., Mcginty, J. F., Gerfen, C. R. & Bloom, F. E. The organization of projections from the cortex, amygdala, and hypothalamus to the nucleus of the solitary tract in rat. J. Comp. Neurol. 224, 1–24 (1984).
Berendse, H. W. & Groenewegen, H. J. Restricted cortical termination fields of the midline and intralaminar thalamic nuclei in the rat. Neuroscience 42, 73–102 (1991).
Vertes, R. P. & Hoover, W. B. Projections of the paraventricular and paratenial nuclei of the dorsal midline thalamus in the rat. J. Comp. Neurol. 508, 212–237 (2008).
Erben, L., He, M.-X., Laeremans, A., Park, E. & Buonanno, A. A novel ultrasensitive in situ hybridization approach to detect short sequences and splice variants with cellular resolution. Mol. Neurobiol. 55, 6169–6181 (2018).
Erben, L. & Buonanno, A. Detection and quantification of multiple RNA sequences using emerging ultrasensitive fluorescent in situ hybridization techniques. Curr. Protoc. Neurosci. 87, e63 (2019).
Guo, Z. V. et al. Procedures for behavioral experiments in head-fixed mice. PLoS One 9, e88678 (2014).
Watabe-Uchida, M., Zhu, L., Ogawa, S. K., Vamanrao, A. & Uchida, N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron 74, 858–873 (2012).
Acknowledgements
We thank the Section for Instrumentation of the NIMH for the design and manufacture of the thermal chamber and the NIMH Transgenic Core for the ovariectomized C57BL/6NJ mice. In addition, we thank F. Do Monte (UT Health) and F. Matyas (Hungarian Academy of Sciences) for their comments on the manuscript. This work was supported by the NIMH Intramural Research Program (1ZIAMH002950, to M.A.P.).
Author information
Authors and Affiliations
Contributions
C.G., Y.L. and M.A.P. performed all experiments in this study. J.M., S.R.-G. and V.R. assisted with stereotaxic injections and immunohistochemical studies. C.R. and K.D. provided critical reagents and suggestions. M.A.P. designed the study. C.G. and M.A.P. analyzed the data, interpreted results and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Neuroscience thanks Ivan de Araujo, Kay Tye, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
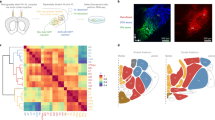
Extended Data Fig. 1 Validation of our CreOFF vectors.
Top: Representative images from an RNAscope ISH experiment used to validate our FlexOFF (a.k.a. CreOFF) construct (produced by Vector Biolabs). AAV2-EF1a-FLEX/OFF-hChR2(H134R)-eYFP was injected in the PVT of a Drd2-Cre mouse and allowed to express for two weeks. Notice that ChR2-eYFP is almost exclusively (~ 95%) expressed in Drd2-negative cells (arrows). Data from 1 mouse, with the experiment independently repeated twice. Similar results were obtained on both occasions. Bottom: Representative images from an immunohistochemistry experiment used to validate our DO (CreOFF) construct (cloned and packaged by Charu Ramakrishnan). AAV9-Syn-DO-GCaMP6s was injected in the PVT of a Drd2-Cre mouse and allowed to express for two weeks. An antibody against D2R (see Methods) was used to label D2R+ regions of the PVT. Notice that GCaMP6s expression is selectively observed in areas where D2 labeling is low or absent (arrows). Data from 1 mouse.
Extended Data Fig. 2 Anatomical projections from Type I and Type II neurons of the PVT.
a, Representative images showing the distribution of axonal projections from Type I neurons of the pPVT (top) and Type II neurons of the aPVT (bottom) across three major PVT targets: the mPFC, the NAc and the amygdala (data from 3 mice). b, Representative images showing the distribution of the axonal projections from Type I (top) and Type II (bottom) neurons of the aPVT across the mPFC, the NAc and the amygdala. Notice that since Type I neurons are rather scarce in the aPVT, the efferent projections from these neurons are sparse and more difficult to resolve. Data from 2 mice. c, Representative images show the distribution of the axonal projections from Type I (top) and Type II (bottom) neurons of the pPVT across the mPFC, the NAc and the amygdala. Because only a few Type II neurons populate the pPVT, the efferent projections from these neurons are sparse and not as dense when compared to those of Type I neurons. Data from 2 mice. Arrowheads indicate regions selectively innervated by Type I neurons, whereas arrows indicate regions selectively innervated by Type II neurons. Magnifications of the areas depicted by the yellow boxes are shown as inserts. Anterograde tracing experiments were independently repeated three times, with similar results obtained across repetitions.
Extended Data Fig. 3 Type II neurons selectively express Galanin (Gal).
a, Schematics of the Spatial Search tool used to identify experiments in which PVT projections to the IL were detected (Mouse Brain Connectivity Atlas of the Allen Brain Institute). b, Sample images from Experiment # 204908781 (Gal-Cre:KI87) demonstrating that the axonal projections of Gal+ neurons of the PVT resemble those of Type II neurons (Extended Data Fig. 2). c, Representative images from our multiplexed RNAscope experiment aimed at detecting Gal (red) and Drd2 (green) expression in the aPVT (left). Insert shows magnified portion depicted by the yellow box (right). d, Representative images from our multiplexed RNAscope experiment aimed at detecting Gal (red) and Drd2 (green) expression in the pPVT. e, Same as c with counterbalanced channels. f, Same as d with counterbalanced channels. g, Quantification of Gal and Drd2 expression across six different bregma locations, n = 5 sections per bregma location (data from 4 mice). h, Box plot shows percent colocalization for both Drd2 (red) and Gal (black) across all bregma locations, n = 5 sections per bregma location (data from 4 mice). Multiplexed RNAscope experiments for simultaneous detection of Gal and Drd2 in the PVT were independently repeated four times, with probe channels counterbalanced across repetitions. All independent repetitions yielded similar results. Box chart legend: box is defined by 25th, 75th percentiles, whiskers are determined by 5th and 95th percentiles, and mean is depicted by the square symbol. i, Schematic of the stereotaxic injection used to selectively target expression of GCaMP6s to Gal+ neurons of the aPVT of Gal-Cre mice. j, Average GCaMP6s responses to footshocks from Gal+ neurons of the PVT (n = 2 mice). Footshock duration depicted by the blue line above the trace. k, Heatmap of dF/F for all individual trials. Data shown as mean ± s.e.m.
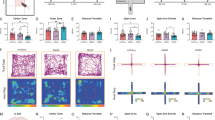
Extended Data Fig. 4 Type I and Type II neurons of the PVT display opposite changes in spontaneous firing in response to an aversive stimulus.
a, Left: schematic of the approach for individually assessing the effect of footshocks on the spontaneous activity of Type I and Type II PVT neurons. Right: sample recordings highlighting the different resting states observed for PVT neurons. Type I and Type II PVT neurons varied in their proportion of silent (top), tonic firing (middle), and burst firing (bottom) cells. b, Pie charts summarizing the proportion of silent (white), tonic firing (red), and burst firing (pink) neurons across naïve and shocked mice for both Type I and Type II subclasses. Naïve: Type I, n = 29 neurons from 4 mice; Type II, n = 32 neurons from 4 mice. Shocked: Type I, n = 22 neurons from 3 mice; Type II, n = 19 neurons from 3 mice.
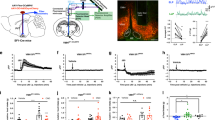
Extended Data Fig. 5 Pseudo-typed rabies tracing of monosynaptic inputs onto ILPVT neurons.
a, Schematic of the viral vector strategy employed for the pseudo-typed rabies tracing of monosynaptic inputs to ILPVT neurons. b, Representative image of Rabies-GFP and TVA-mCherry double-labelled cells illustrate the location of rabies starter cells. c, Rabies-GFP (retrogradely-labelled) neurons are observed throughout the aPVT, which is enriched with Type II PVT neurons (Fig. 1). Rabies-GFP neurons were rarely observed in the pPVT and restricted to the most dorsal part of the pPVT, a region of the pPVT dominated by Type II PVT neurons. These findings demonstrate that IL-projection neurons of the PVT are monosynaptically connected to ILPVT neurons, highlighting the presence of a PVT-IL thalamo-corticothalamic loop (data from 2 mice). This experiment was independently repeated twice with similar results.
Extended Data Fig. 6 Fiber photometry imaging of GCaMP6s fluorescence from the terminals of PVT neurons in the IL.
a, Schematic of the viral vector strategy and optical fiber placement used for fiber photometry imaging of GCaMP6s fluorescence from the IL terminals of Type II PVT neurons. b, Left: average GCaMP6s response to footshocks from IL terminals of Type II PVT neurons. Footshock duration depicted by the red line above the trace, n = 6 mice. Right: quantification of GCaMP6s responses to footshocks. AUC, Before, -0.0015 ± 0.00076; After, -5.82 ± 0.39, n = 6 mice, **P=0.0.00003, two-sided Paired sample t-test. c, Left: average GCaMP6s response to social interaction from the IL terminals of Type II PVT neurons. The duration of the interaction bouts is depicted by the red line above the trace, n = 6 mice. Right: quantification of GCaMP6s responses to social interaction. AUC, Before, -0.00072 ± 0.008; After, -36.33 ± 10.48, n = 6 mice, **P=0.0.00003, two-sided Paired sample t-test. Box chart legend: box is defined by 25th, 75th percentiles, whiskers are determined by 5th and 95th percentiles, and mean is depicted by the square symbol. Data shown as mean ± s.e.m.
Extended Data Fig. 7 Optogenetic silencing of PVT input to the IL does not alter cortical responses to salient stimuli.
a, Top: heatmap of average GCaMP6s responses to footshocks from individual halorhodopsin-expressing subjects. Bottom left: average GCaMP6s response from ILPVT neurons in halorhodopsin-expressing animals subjected to footshocks in the presence (red) and absence (black) of light stimulation (561 nm at 20 Hz). Footshock duration is depicted by the blue line above the trace, n = 7 mice. Bottom right: quantification of footshock-evoked changes in GCaMP6s fluorescence in ILPVT neurons in the presence and absence of light stimulation. AUC, Off_Before, -0.05 ± 0.03; Off_After, -52.11 ± 16.54; On_Before, 0.02 ± 0.02; On_After, -67.56 ± 20.74, n = 7 mice; F(3,22) = 6.84, two-way ANOVA followed by Tukey’s test. Group comparisons: Off_Before vs Off_After, P=0.07; On_Before vs On_After, **P=0.007; Off_After vs On_After, P=0.85. b, Top: heatmap of average GCaMP6s responses to social interaction from individual halorhodopsin-expressing subjects. Bottom left: average GCaMP6s response from ILPVT neurons in halorhodopsin-expressing animals subjected to social interaction in the presence (red) and absence (black) of light stimulation. The duration of the interaction bouts is depicted by the blue line above the trace, n = 5 mice. Bottom right: quantification of social interaction-evoked changes in GCaMP6s fluorescence in ILPVT neurons in the presence and absence of light stimulation. AUC, Off_Before, -0.71 ± 13.35; Off_After, -184.78 ± 47.13; On_Before, 15.42 ± 17.02; On_After, -149.26 ± 55.37, n = 5 mice; F(3,16) = 7.22, two-way ANOVA followed by Tukey’s test. Group comparisons: Off_Before vs Off_After, *P=0.02; On_Before vs On_After, *P=0.03; Off_After vs On_After, P=0.92. Box chart legend: box is defined by 25th, 75th percentiles, whiskers are determined by 5th and 95th percentiles, and mean is depicted by the square symbol. Data shown as mean ± s.e.m.
Extended Data Fig. 8 Schematic of the rostro-caudal distribution of Type I and Type II neurons of the PVT.
a, Sagittal view of the PVT illustrating the distribution of Type I (gray) and Type II (red) neuronal cell types. Dashed lines indicate (from left to right) the aPVT, middle PVT (mPVT) and pPVT. b, Coronal view of the distribution of Type I and Type II neuronal cell types across different anteroposterior regions of the PVT. Notice that similar proportions of Type I and Type II PVT neurons populate the mPVT.
Extended Data Fig. 9 Gal expression in the PVT is relatively lower compared to the hypothalamus.
Sagittal (top) and coronal (bottom) sample images from two independent Gal ISH experiments from the Mouse Gene Expression Atlas of the Allen Brain Institute. Notice that Gal expression in the PVT (yellow rectangle) is appreciably lower when compared to expression observed in the hypothalamus (arrow).
Extended Data Fig. 10 Optical fiber placements.
a, Top: Optical fiber placements for the GCaMP6s imaging experiment of Type I neurons of the PVT shown in Fig. 1h–k. Bottom: Optical fiber placements for the GCaMP6s imaging experiment of Type II neurons shown in Fig. 1h–k. b, Optical fiber placement for the GCaMP6s imaging experiment from the terminals of Type I neurons of the PVT in the IL shown in Extended Data Fig. 6. c, Left: Optical fiber placements for the ChrimsonR-expressing mice used for the combined optogenetic and GCaMP6s experiment shown in Fig. 3. Right: Optical fiber placements for the mCherry-expressing mice used for the combined optogenetic and GCaMP6s experiment shown in Fig. 3. d, Optical fiber placements for the halorhodopsin-expressing mice used for the combined optogenetic and GCaMP6s experiment shown in Extended Data Fig. 7. e, Left: Optical fiber placement for combined polysomnography and fiber photometry imaging of the terminals of Type II PVT neurons in the IL shown in Fig. 4. Right: Optical fiber placement for the combined polysomnography and fiber photometry imaging of ILPVT neurons shown in Fig. 5. f, Top left: Optical fiber placements for the GCaMP6s imaging experiment from ILPVT neurons shown in Fig. 6a–e. Top right: Optical fiber placements for the mCherry-expressing mice used for the combined optogenetic and pupillometry experiment shown in Fig. 6f–i. Bottom left: Optical fiber placements for the ChrimsonR-expressing mice used for the combined optogenetic and pupillometry experiment shown in Fig. 6f–i. Bottom right: Optical fiber placements for the Halo-expressing mice used for the combined optogenetic and pupillometry experiment shown in Fig. 6f–i. All circles depict the lowest position of the optical fibers for each subject.
Supplementary information
Rights and permissions
About this article
Cite this article
Gao, C., Leng, Y., Ma, J. et al. Two genetically, anatomically and functionally distinct cell types segregate across anteroposterior axis of paraventricular thalamus. Nat Neurosci 23, 217–228 (2020). https://doi.org/10.1038/s41593-019-0572-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-019-0572-3
This article is cited by
-
A midbrain GABAergic circuit constrains wakefulness in a mouse model of stress
Nature Communications (2024)
-
Modulation of learning safety signals by acute stress: paraventricular thalamus and prefrontal inhibition
Neuropsychopharmacology (2024)
-
Activation patterns in male and female forebrain circuitries during food consumption under novelty
Brain Structure and Function (2024)
-
A phylogenetically-conserved axis of thalamocortical connectivity in the human brain
Nature Communications (2023)
-
Thalamic subnetworks as units of function
Nature Neuroscience (2022)