Abstract
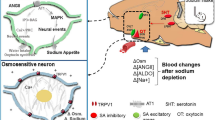
Sodium appetite is a powerful form of motivation that can drive ingestion of high, yet aversive concentrations of sodium in animals that are depleted of sodium. However, in normal conditions, sodium appetite is suppressed to prevent homeostatic deviations. Although molecular and neural mechanisms underlying the stimulation of sodium appetite have received much attention recently, mechanisms that inhibit sodium appetite remain largely obscure. Here we report that serotonin 2c receptor (Htr2c)-expressing neurons in the lateral parabrachial nucleus (LPBNHtr2c neurons) inhibit sodium appetite. Activity of these neurons is regulated by bodily sodium content, and their activation can rapidly suppress sodium intake. Conversely, inhibition of these neurons specifically drives sodium appetite, even during euvolemic conditions. Notably, the physiological role of Htr2c expressed by LPBN neurons is to disinhibit sodium appetite. Our results suggest that LPBNHtr2c neurons act as a brake against sodium appetite and that their alleviation is required for the full manifestation of sodium appetite.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Code availability
Custom code used in this study is available from the corresponding authors upon reasonable request.
References
Wagman, W. Sodium chloride deprivation: development of sodium chloride as a reinforcement. Science 140, 1403–1404 (1963).
Matsuda, T.et al. Distinct neural mechanisms for the control of thirst and salt appetite in the subfornical organ. Nat. Neurosci. 20, 230–241 (2017).
Jarvie, B. C. & Palmiter, R. D. HSD2 neurons in the hindbrain drive sodium appetite. Nat. Neurosci. 20, 167–169 (2017).
Resch, J. M. et al. Aldosterone-sensing neurons in the NTS exhibit state-dependent pacemaker activity and drive sodium appetite via synergy with angiotensin II signaling. Neuron 96, 190–206 (2017).
Oka, Y., Butnaru, M., von Buchholtz, L., Ryba, N. J. & Zuker, C. S. High salt recruits aversive taste pathways. Nature 494, 472–475 (2013).
de Oliveira, L. B., Callera, J. C., De Luca, L. A. Jr, Colombari, D. S. & Menani, J. V. GABAergic mechanisms of the lateral parabrachial nucleus on sodium appetite. Brain Res. Bull. 73, 238–247 (2007).
De Gobbi, J. I. et al. Non-NMDA receptors in the lateral parabrachial nucleus modulate sodium appetite. Brain Res. 1301, 44–51 (2009).
Johnson, A. K. & Thunhorst, R. L. The neuroendocrinology of thirst and salt appetite: visceral sensory signals and mechanisms of central integration. Front. Neuroendocrinol. 18, 292–353 (1997).
Scalera, G., Spector, A. C. & Norgren, R. Excitotoxic lesions of the parabrachial nuclei prevent conditioned taste aversions and sodium appetite in rats. Behav. Neurosci. 109, 997–1008 (1995).
Geerling, J. C. et al. FoxP2 expression defines dorsolateral pontine neurons activated by sodium deprivation. Brain Res. 1375, 19–27 (2011).
Castro, L. et al. Central 5-HT2B/2C and 5-HT3 receptor stimulation decreases salt intake in sodium-depleted rats. Brain Res. 981, 151–159 (2003).
Gentili, L., Saija, A., Luchetti, G. & Massi, M. Effect of the 5-HT2 antagonist ketanserin on salt appetite in the rat. Pharmacol. Biochem. Behav. 39, 171–176 (1991).
Menani, J. V., Thunhorst, R. L. & Johnson, A. K. Lateral parabrachial nucleus and serotonergic mechanisms in the control of salt appetite in rats. Am. J. Physiol. 270, R162–R168 (1996).
De Gobbi, J. I. et al. 5-HT2 and 5-HT3 receptors in the lateral parabrachial nucleus mediate opposite effects on sodium intake. Neuroscience 146, 1453–1461 (2007).
Yamamoto, T. et al. c-Fos expression in the parabrachial nucleus after ingestion of sodium chloride in the rat. Neuroreport 4, 1223–1226 (1993).
Geerling, J. C. & Loewy, A. D. Sodium deprivation and salt intake activate separate neuronal subpopulations in the nucleus of the solitary tract and the parabrachial complex. J. Comp. Neurol. 504, 379–403 (2007).
Ryan, P. J., Ross, S. I., Campos, C. A., Derkach, V. A. & Palmiter, R. D. Oxytocin-receptor-expressing neurons in the parabrachial nucleus regulate fluid intake. Nat. Neurosci. 20, 1722–1733 (2017).
Donnelly, M. L. et al. Analysis of the aphthovirus 2A/2B polyprotein ‘cleavage’ mechanism indicates not a proteolytic reaction, but a novel translational effect: a putative ribosomal ‘skip’. J. Gen. Virol. 82, 1013–1025 (2001).
Carter, M. E., Soden, M. E., Zweifel, L. S. & Palmiter, R. D. Genetic identification of a neural circuit that suppresses appetite. Nature 503, 111–114 (2013).
Campos, C. A. et al. Cancer-induced anorexia and malaise are mediated by CGRP neurons in the parabrachial nucleus. Nat. Neurosci. 20, 934–942 (2017).
Campos, C. A., Bowen, A. J., Schwartz, M. W. & Palmiter, R. D. Parabrachial CGRP neurons control meal termination. Cell Metab. 23, 811–820 (2016).
Garfield, A. S. et al. A neural basis for melanocortin-4 receptor-regulated appetite. Nat. Neurosci. 18, 863–871 (2015).
Stricker, E. M. & Verbalis, J. G. Central inhibitory control of sodium appetite in rats: correlation with pituitary oxytocin secretion. Behav. Neurosci. 101, 560 (1987).
Geerling, J. C., Chimenti, P. C. & Loewy, A. D. Phox2b expression in the aldosterone-sensitive HSD2 neurons of the NTS. Brain Res. 1226, 82–88 (2008).
Kang, B. J. et al. Central nervous system distribution of the transcription factor Phox2b in the adult rat. J. Comp. Neurol. 503, 627–641 (2007).
Hashimoto, K., Obata, K. & Ogawa, H. Characterization of parabrachial subnuclei in mice with regard to salt tastants: possible independence of taste relay from visceral processing. Chem. Senses 34, 253–267 (2009).
Biondolillo, J. W., Williams, L. A. & King, M. S. Blocking glutamate receptors in the waist area of the parabrachial nucleus decreases taste reactivity behaviors in conscious rats. Chem. Senses 34, 221–230 (2009).
Chandrashekar, J. et al. The cells and peripheral representation of sodium taste in mice. Nature 464, 297–301 (2010).
Zardetto-Smith, A. M., Beltz, T. G. & Johnson, A. K. Role of the central nucleus of the amygdala and bed nucleus of the stria terminalis in experimentally-induced salt appetite. Brain Res. 645, 123–134 (1994).
Galaverna, O., De Luca, L. A. Jr, Schulkin, J., Yao, S. Z. & Epstein, A. N. Deficits in NaCl ingestion after damage to the central nucleus of the amygdala in the rat. Brain Res. Bull. 28, 89–98 (1992).
Dong, H. W., Petrovich, G. D. & Swanson, L. W. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res. Rev. 38, 192–246 (2001).
Dong, H. W., Petrovich, G. D., Watts, A. G. & Swanson, L. W. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. J. Comp. Neurol. 436, 430–455 (2001).
Lee, S. et al. Chemosensory modulation of neural circuits for sodium appetite. Nature 568, 93–97 (2019).
Chen, Y., Baez, M. & Yu, L. Functional coupling of the 5-HT2C serotonin receptor to G proteins in Xenopus oocytes. Neurosci. Lett. 179, 100–102 (1994).
Lucaites, V. L., Nelson, D. L., Wainscott, D. B. & Baez, M. Receptor subtype and density determine the coupling repertoire of the 5-HT2 receptor subfamily. Life Sci. 59, 1081–1095 (1996).
Kurachi, Y., Tung, R. T., Ito, H. & Nakajima, T. G protein activation of cardiac muscarinic K+ channels. Prog. Neurobiol. 39, 229–246 (1992).
Petrov, T., Krukoff, T. L. & Jhamandas, J. H. The hypothalamic paraventricular and lateral parabrachial nuclei receive collaterals from raphe nucleus neurons: a combined double retrograde and immunocytochemical study. J. Comp. Neurol. 318, 18–26 (1992).
Bang, S. J., Jensen, P., Dymecki, S. M. & Commons, K. G. Projections and interconnections of genetically defined serotonin neurons in mice. Eur. J. Neurosci. 35, 85–96 (2012).
Smits, J. F., van Essen, H. & Struyker-Boudier, H. A. Serotonin-mediated cardiovascular responses to electrical stimulation of the raphe nuclei in the rat. Life Sci. 23, 173–178 (1978).
Berglund, E. D. et al. Serotonin 2C receptors in pro-opiomelanocortin neurons regulate energy and glucose homeostasis. J. Clin. Invest. 123, 5061–5070 (2013).
Acknowledgements
We thank G.S.B. Suh (KAIST) for helpful comments on the manuscript, S.-H. Lee (KAIST) for technical help with viral tracing experiments and D. Kim (KAIST) for technical help with imaging. This work was supported by grants from the Samsung Science & Technology Foundation (SSTF-BA1901-11 to J.-W.S.), the KAIST Future Systems Health Care Project (to J.-W.S.), the KAIST Venture Research Program for Graduate and PhD students (to S.P.), the American Heart Association (16SDG27260001 to C.L.) and the National Institutes of Health (R01 DK114036 to C.L. and R01 DK100699, R01 DK119169, DK119130 5830 to K.W.W.). S.P. was supported in part by stipends from the National Research Foundation of Korea (NRF-2015M3A9E7029177 and NRF-2016R1C1B2006614 to J.-W.S.).
Author information
Authors and Affiliations
Contributions
S.P. and J.-W.S. designed the experiments. S.P. conducted experiments and analyzed data. C.L. and K.W.W. provided reagents and expertise. C.L. generated and validated Htr2c-2A-iCre mice. S.P. and J.-W.S. wrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Neuroscience thanks Yuki Oka and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 LPBN neurons respond to hypertonic saline.
Hypertonic saline-loaded mice show more Fos in the LPBN than saline-loaded controls. Two-tailed Mann-Whitney test, Saline N = 5 mice, Hypertonic saline N =5 mice. P = 0.0317. All data represented as mean ± s.e.m. *P < 0.05.
Extended Data Fig. 2 Generation of an Htr2c-2A-iCre mouse line.
(a) Htr2c-2A-iCre knock-in mice in which the coding sequences of Htr2c and iCre are linked by a viral peptide bridge 2A sequence. (b) Detection of wildtype and targeted Htr2c alleles by PCR genotyping. Similar results were obtained with at least 3 independent replications. (c) Immunohistochemical analysis of Htr2c-2A-iCre activity using a tdTomato reporter. Note high levels of tdTomato expression in the choroid plexus (CP). CC = corpus callosum. Similar results were obtained with at least 3 mice. Scale bar = 100 μm Source data.
Extended Data Fig. 3 EYFP-expressing LPBNHtr2c neurons are inhibited during sodium depletion.
(a) Cell-attached recordings taken from Htr2c-2A-iCre mice injected with AAV5-E1fa-DIO-EYFP. (b) Representative cell-attached recording of LPBNHtr2c neurons after 7 days of control diet, or 7 days of sodium-deficient diet. (c) Firing frequency of LPBNHtr2c neurons between control diet-fed mice (left) and sodium-deficient diet-fed mice (right). Two-tailed Mann-Whitney test. Control diet n = 21 cells, sodium-deficient diet n = 24 cells. Control diet 1.2 ± 0.3 Hz, Sodium-deficient diet 0.4 ± 0.2 Hz, P = 0.007. All data represented as mean ± s.e.m. **P < 0.01.
Extended Data Fig. 4 CNO injections in AAV-DIO-mCherry-injected controls do not affect food intake, water intake or 300 mM NaCl intake.
(a) Htr2c-2A-iCre mice injected with AAV2-hSyn-DIO-mCherry showed no changes in food intake, body weight or 300 mM NaCl intake. (b) No changes in water intake or 300 mM NaCl intake during sodium depletion. N = 5 mice. Water intake during sodium depletion: Two-Way Repeated Measures ANOVA: time (F(4, 16) = 20.52, P < 0.0001), treatment (F(1, 4) = 0.5421, P = 0.5024), interaction (F(4, 16) = 0.6448, P = 0.6385). 300 mM NaCl intake during sodium depletion: Two-Way Repeated Measures ANOVA: time (F(4,16) = 108.1, P < 0.0001), treatment (F(1, 4) = 0.1128, P = 0.7538), interaction (F(4, 16) = 0.3142, P = 0.8643). (c) No changes in food intake or body weight when using a control diet. N = 5 mice. Control diet food intake: Two-Way Repeated Measures ANOVA: time (F(3,12) = 223.9, P < 0.0001), treatment (F(1, 4) = 0.2222, P = 0.6619), interaction (F(3,12) = 2.689, P = 0.0924). All data represented as mean ± s.e.m.
Extended Data Fig. 5 LPBNHtr2c neuron activation can cause non-sodium related effects.
(a) Unilateral injection of AAV5-hSyn-DIO-hM3Dq into the LPBN of Htr2c-2A-iCre mice. (b) Activation of LPBNHtr2c neurons decreased water and 300 mM NaCl intake during dehydration in a two-bottle choice assay. N =11. Water intake during dehydration: Two-Way Repeated Measures ANOVA: time (F(4,40) = 67.06, P < 0.0001), treatment (F(1, 10) = 8.707, P = 0.0145), interaction (F(4, 40) = 6.329, P = 0.0005). Post hoc tests using the Bonferroni correction. 300 mM NaCl intake during dehydration: Two-Way Repeated Measures ANOVA: time (F(4,40) = 31.38, P < 0.0001), treatment (F(1, 10) = 17.76 P = 0.0018), interaction (F(4, 40) = 14.06 P = 0.0001). (c) Activation of LPBNHtr2c neurons decreased food during a fast refeeding regardless of sodium content. N = 11. Control diet food intake (left): Two-Way Repeated Measures ANOVA: time (F(3,30) = 111.0, P < 0.0001), treatment (F(1, 10) = 51.2, P < 0.0001), interaction (F(3, 30) = 32.00, P < 0.0001). Sodium-deficient diet food intake (right): Two-Way Repeated Measures ANOVA: time (F(3,30) = 50.59, P < 0.0001), treatment (F(1, 10) = 27.74, P = 0.0004), interaction (F(3, 30) = 18.81, P < 0.0001). All post hoc tests using the Bonferroni correction. All data represented as mean ± s.e.m. ****P < 0.0001.
Extended Data Fig. 6 LPBNHtr2c neurons colocalise with CGRP.
(a) Schematic of experiment. Unilateral injection of AAV5-EF1a-DIO -EYFP into the LPBN of Htr2c-2A-iCre mice to visualise LPBNHtr2c neurons (left). Colocalisation of EYFP labelled LPBNHtr2c neurons with CGRP (right). Cyan = EYFP, Magenta = CGRP, Blue = DAPI. Scale bar = 100 μm. (b) Estimation of colocalisation between LPBNHtr2c neurons and CGRP neurons. 3 slices taken from each mice. N = 7 mice.
Extended Data Fig. 7 Downstream circuitry of LPBNHtr2c neurons.
(a) Axonal projections visualised by unilateral injection of AAV5-EF1a-DIO-EYFP into the LPBN of Htr2c-2A-iCre mice. aca = anterior commissure (anterior part), acp = anterior commissure (posterior part), dBNST = bed nucleus of the stria terminalis (lateral division), fr = fasciculus retroflexus, ml = medial lemniscus, MnPO = median preoptic nucleus, opt = optic tract, PVH = paraventricular nucleus of the hypothalamus, scp = superior cerebellar peduncle, SPF = subparafascicular thalamus, st = stria terminalis, vBNST = bed nucleus of the stria terminalis (ventral part), VPPC = ventral posterior thalamus (parvicellular part). Scale bar = 400 μm. N = 7 mice. (b) Schematic of experiment. Bilateral injection of AAV2-DIO-hChR2 (H134R)-p2A-mCherry into the LPBN of Htr2c-2A-iCre mice (left). Voltage-clamp recordings taken at downstream sites (traces on the right). Blue rectangles indicate times of photostimulation.
Extended Data Fig. 8 Illustration showing the approximate spatial distribution of mCPP-responsive neurons within the LPBN.
C = central LPBN, D = dorsal LPBN, E = external LPBN, I = internal LPBN, KF = Kolliker’s Fuse, V = ventral LPBN.
Extended Data Fig. 9 Hyperpolarisation of LPBNHtr2c neurons is not dependent on Htr1b and is postsynaptic.
(a) Representative current clamp recording of an LPBNHtr2c neuron pre-treated with SB216641 (200 nM). Hyperpolarisation in response to mCPP persists in presence of SB216641. Arrows indicate time at which current steps were applied. (b) Voltage deflections in response to hyperpolarising currents from the same neuron, showing decreased input resistance in response to mCPP. Current steps in trace made in 10 pA increments from -50 pA to 0 pA. (c) Representative current clamp recording of an LPBNHtr2c neuron pre-treated with TTX (500 nM), kynurenic acid (KA, 1 mM) and picrotoxin (PTX, 50 μM), showing hyperpolarisation in response to mCPP. Arrows indicate time at which current steps were applied. (d) Voltage deflections in response to hyperpolarising currents from the same neuron, showing decreased input resistance in response to mCPP. Current steps in trace made in 10 pA increments from -50 pA to 0 pA. (e) Representative current clamp recording of an LPBNHtr2c neuron from a fasted mouse (18 hours), showing hyperpolarisation in response to mCPP. Arrows indicate time at which current steps were applied. (f) Voltage deflections in response to hyperpolarising currents from the same neuron, showing decreased input resistance in response to mCPP. Current steps in trace made in 10 pA increments from -50 pA to 0 pA. (g) Graph summarising change in membrane potential in response to mCPP under conditions tested. Neither pre-treatment with SB216641, Synaptic blockers nor recordings from fasted mice changed the magnitude of response to mCPP. mCPP n = 10 cells, SB216641 n = 5 cells, Synaptic block n = 3 cells, Fasted n = 8 cells. Data represented as mean ± s.e.m.
Extended Data Fig. 10 Serotoninergic projections to the LPBN.
(a) Schematic of experiment. Unilateral injection of Lumafluor retrobeads into the LPBN of wildtype mice to visualise upstream neurons. (b) Arrows indicate colocalised cells. Aq = aqueduct, DR = dorsal raphe. Scale bar = 200 μm. N = 6 mice (upper). MnR = median raphe. Scale bar = 100 μm. N = 6 mice (lower).
Supplementary information
Supplementary Information
Supplementary Table 1: Adjusted P values for post hoc comparisons.
Source data
Source Data Extended Data Fig. 2
Full gel picture for Extended Data Fig. 2b.
Rights and permissions
About this article
Cite this article
Park, S., Williams, K.W., Liu, C. et al. A neural basis for tonic suppression of sodium appetite. Nat Neurosci 23, 423–432 (2020). https://doi.org/10.1038/s41593-019-0573-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-019-0573-2
This article is cited by
-
Molecular neurobiological markers in the onset of sodium appetite
Scientific Reports (2022)
-
Sodium intake regulation
Nature Reviews Neuroscience (2020)