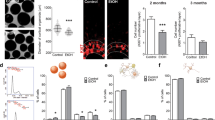
Abstract
Learning disabilities are hallmarks of congenital conditions caused by prenatal exposure to harmful agents. These include fetal alcohol spectrum disorders (FASDs) with a wide range of cognitive deficiencies, including impaired motor skill development. Although these effects have been well characterized, the molecular effects that bring about these behavioral consequences remain to be determined. We previously found that the acute molecular responses to alcohol in the embryonic brain are stochastic, varying among neural progenitor cells. However, the pathophysiological consequences stemming from these heterogeneous responses remain unknown. Here we show that acute responses to alcohol in progenitor cells altered gene expression in their descendant neurons. Among the altered genes, an increase of the calcium-activated potassium channel Kcnn2 in the motor cortex correlated with motor learning deficits in a mouse model of FASD. Pharmacologic blockade of Kcnn2 improves these learning deficits, suggesting Kcnn2 blockers as a new intervention for learning disabilities in FASD.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All raw data and processed data of RNA sequencing are available (GSE141714). Other data are available from the authors upon reasonable request and with permission of the Children’s National Institutional Review Board.
Code availability
Codes are available from the authors upon reasonable request and with permission of the Children’s National Institutional Review Board.
References
Sakurai, T. et al. Converging models of schizophrenia-network alterations of prefrontal cortex underlying cognitive impairments. Prog. Neurobiol. 134, 178–201 (2015).
Thompson, B. L., Levitt, P. & Stanwood, G. D. Prenatal exposure to drugs: effects on brain development and implications for policy and education. Nat. Rev. Neurosci. 10, 303–312 (2009).
Grandjean, P. & Landrigan, P. J. Developmental neurotoxicity of industrial chemicals. Lancet 368, 2167–2178 (2006).
May, P. A. et al. Prevalence of fetal alcohol spectrum disorders in 4 US communities. JAMA 319, 474–482 (2018).
Diamond, A. & Lee, E. Y. Inability of five-month-old infants to retrieve a contiguous object: a failure of conceptual understanding or of control of action? Child Dev. 71, 1477–1494 (2000).
Streissguth, A. P., Barr, H. M. & Sampson, P. D. Moderate prenatal alcohol exposure: effects on child IQ and learning problems at age 7 1/2 years. Alcohol. Clin. Exp. Res. 14, 662–669 (1990).
Hashimoto-Torii, K. et al. Roles of heat shock factor 1 in neuronal response to fetal environmental risks and its relevance to brain disorders. Neuron 82, 560–572 (2014).
Torii, M. et al. Detection of vulnerable neurons damaged by environmental insults in utero. Proc. Natl Acad. Sci. USA 114, 2367–2372 (2017).
Hashimoto-Torii, K., Kawasawa, Y. I., Kuhn, A. & Rakic, P. Combined transcriptome analysis of fetal human and mouse cerebral cortex exposed to alcohol. Proc. Natl Acad. Sci. USA 108, 4212–4217 (2011).
Ishii, S. et al. Variations in brain defects result from cellular mosaicism in the activation of heat shock signalling. Nat. Commun. 8, 15157 (2017).
Akerfelt, M., Morimoto, R. I. & Sistonen, L. Heat shock factors: integrators of cell stress, development and lifespan. Nat. Rev. Mol. Cell Biol. 11, 545–555 (2010).
Ishii, S. & Hashimoto-Torii, K. Impact of prenatal environmental stress on cortical development. Front. Cell. Neurosci. 9, 207 (2015).
El Fatimy, R. et al. Heat shock factor 2 is a stress-responsive mediator of neuronal migration defects in models of fetal alcohol syndrome. EMBO Mol. Med. 6, 1043–1061 (2014).
Lämke, J., Brzezinka, K., Altmann, S. & Bäurle, I. A hit-and-run heat shock factor governs sustained histone methylation and transcriptional stress memory. EMBO J. 35, 162–175 (2016).
Bayer, S. A. & Altman, J. Neocortical Development. vol. 1 (Raven Press, 1991).
Molyneaux, B. J., Arlotta, P., Menezes, J. R. L. & Macklis, J. D. Neuronal subtype specification in the cerebral cortex. Nat. Rev. Neurosci. 8, 427–437 (2007).
Tan, X. & Shi, S.-H. Neocortical neurogenesis and neuronal migration. Wiley Interdiscip. Rev. Dev. Biol. 2, 443–459 (2013).
Gupta, A., Tsai, L.-H. & Wynshaw-Boris, A. Life is a journey: a genetic look at neocortical development. Nat. Rev. Genet. 3, 342–355 (2002).
Rash, B. G. & Grove, E. A. Area and layer patterning in the developing cerebral cortex. Curr. Opin. Neurobiol. 16, 25–34 (2006).
Marzban, H. et al. Cellular commitment in the developing cerebellum. Front. Cell. Neurosci. 8, 450 (2014).
Heck, D. H., Roy, S., Xie, N. & Waters, R. S. Prenatal alcohol exposure delays acquisition and use of skilled reaching movements in juvenile rats. Physiol. Behav. 94, 540–544 (2008).
Amos-Kroohs, R. M. et al. Abnormal eating behaviors are common in children with fetal alcohol spectrum disorder. J. Pediatr. 169, 194–200.e1 (2016).
Padmashri, R., Reiner, B. C., Suresh, A., Spartz, E. & Dunaevsky, A. Altered structural and functional synaptic plasticity with motor skill learning in a mouse model of fragile X syndrome. J. Neurosci. 33, 19715–19723 (2013).
Xu, T. et al. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature 462, 915–919 (2009).
Esposito, M. S., Capelli, P. & Arber, S. Brainstem nucleus MdV mediates skilled forelimb motor tasks. Nature 508, 351–356 (2014).
Niwa, M. et al. Knockdown of DISC1 by in utero gene transfer disturbs postnatal dopaminergic maturation in the frontal cortex and leads to adult behavioral deficits. Neuron 65, 480–489 (2010).
Tabata, H. & Nakajima, K. Labeling embryonic mouse central nervous system cells by in utero electroporation. Dev. Growth Differ. 50, 507–511 (2008).
Delatour, L. C., Yeh, P. W. & Yeh, H. H. Ethanol exposure in utero disrupts radial migration and pyramidal cell development in the somatosensory cortex. Cereb. Cortex 29, 2125–2139 (2019).
Qiu, S. et al. Single-neuron RNA-Seq: technical feasibility and reproducibility. Front. Genet. 3, 124 (2012).
van den Brink, S. C. et al. Single-cell sequencing reveals dissociation-induced gene expression in tissue subpopulations. Nat. Methods 14, 935–936 (2017).
Kadota, K., Ye, J., Nakai, Y., Terada, T. & Shimizu, K. ROKU: a novel method for identification of tissue-specific genes. BMC Bioinf. 7, 294 (2006).
Geschwind, D. H. & Konopka, G. Neuroscience in the era of functional genomics and systems biology. Nature 461, 908–915 (2009).
Vancassel, S. et al. Plasma fatty acid levels in autistic children. Prostaglandins Leukot. Essent. Fatty Acids 65, 1–7 (2001).
Najmabadi, H. et al. Deep sequencing reveals 50 novel genes for recessive cognitive disorders. Nature 478, 57–63 (2011).
Krishnan, A. et al. Genome-wide prediction and functional characterization of the genetic basis of autism spectrum disorder. Nat. Neurosci. 19, 1454–1462 (2016).
Lin, M. T., Luján, R., Watanabe, M., Adelman, J. P. & Maylie, J. SK2 channel plasticity contributes to LTP at Schaffer collateral-CA1 synapses. Nat. Neurosci. 11, 170–177 (2008).
Pedarzani, P. et al. Tamapin, a venom peptide from the Indian red scorpion (Mesobuthus tamulus) that targets small conductance Ca2+-activated K+ channels and afterhyperpolarization currents in central neurons. J. Biol. Chem. 277, 46101–46109 (2002).
Abel, H. J., Lee, J. C. F., Callaway, J. C. & Foehring, R. C. Relationships between intracellular calcium and afterhyperpolarizations in neocortical pyramidal neurons. J. Neurophysiol. 91, 324–335 (2004).
Bean, B. P. The action potential in mammalian central neurons. Nat. Rev. Neurosci. 8, 451–465 (2007).
Sun, J. et al. UBE3A regulates synaptic plasticity and learning and memory by controlling SK2 channel endocytosis. Cell Rep. 12, 449–461 (2015).
Baudry, M. et al. Ampakines promote spine actin polymerization, long-term potentiation, and learning in a mouse model of Angelman syndrome. Neurobiol. Dis. 47, 210–215 (2012).
Chen, L. et al. SK channel blockade reverses cognitive and motor deficits induced by nigrostriatal dopamine lesions in rats. Int. J. Neuropsychopharmacol. 17, 1295–1306 (2014).
Lam, J., Coleman, N., Garing, A. L. A. & Wulff, H. The therapeutic potential of small-conductance KCa2 channels in neurodegenerative and psychiatric diseases. Expert Opin. Ther. Targets 17, 1203–1220 (2013).
Beggs, J. M. & Plenz, D. Neuronal avalanches in neocortical circuits. J. Neurosci. 23, 11167–11177 (2003).
Iwawaki, T. et al. Transgenic mouse model for imaging of ATF4 translational activation-related cellular stress responses in vivo. Sci. Rep. 7, 46230 (2017).
Oikawa, D., Akai, R., Tokuda, M. & Iwawaki, T. A transgenic mouse model for monitoring oxidative stress. Sci. Rep. 2, 229 (2012).
Disterhoft, J. F. & Oh, M. M. Learning, aging and intrinsic neuronal plasticity. Trends Neurosci. 29, 587–599 (2006).
Strassmaier, T. et al. A novel isoform of SK2 assembles with other SK subunits in mouse brain. J. Biol. Chem. 280, 21231–21236 (2005).
Cholewa-Waclaw, J. et al. The role of epigenetic mechanisms in the regulation of gene expression in the nervous system. J. Neurosci. 36, 11427–11434 (2016).
Ostrea, E. M. et al. Fatty acid ethyl esters in meconium: are they biomarkers of fetal alcohol exposure and effect? Alcohol. Clin. Exp. Res. 30, 1152–1159 (2006).
Hashimoto-Torii, K. et al. Interaction between Reelin and Notch signaling regulates neuronal migration in the cerebral cortex. Neuron 60, 273–284 (2008).
Torii, M. & Levitt, P. Dissociation of corticothalamic and thalamocortical axon targeting by an EphA7-mediated mechanism. Neuron 48, 563–575 (2005).
Raymond, C. S. & Soriano, P. High-efficiency FLP and PhiC31 site-specific recombination in mammalian cells. PLoS One 2, e162 (2007).
Matsuda, T. & Cepko, C. L. Electroporation and RNA interference in the rodent retina in vivo and in vitro. Proc. Natl Acad. Sci. USA 101, 16–22 (2004).
Yang, T. et al. Small-conductance Ca2+-activated potassium channels negatively regulate aldosterone secretion in human adrenocortical cells. Hypertension 68, 785–795 (2016).
Langmead, B., Trapnell, C., Pop, M. & Salzberg, S. L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25 (2009).
Kim, D. et al. Tophat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 14, R36 (2013).
Trapnell, C. et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 28, 511–515 (2010).
Anders, S. & Huber, W. Differential expression analysis for sequence count data. Genome Biol. 11, R106 (2010).
Langfelder, P. & Horvath, S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinf. 9, 559 (2008).
Langfelder, P. & Horvath, S. Fast R functions for robust correlations and hierarchical clustering. J. Stat. Softw. 46, i11 (2012).
Bindea, G. et al. ClueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 25, 1091–1093 (2009).
Takeuchi, K. et al. Serotonin-2C receptor involved serotonin-induced Ca2+ mobilisations in neuronal progenitors and neurons in rat suprachiasmatic nucleus. Sci. Rep. 4, 4106 (2014).
Zonouzi, M. et al. GABAergic regulation of cerebellar NG2 cell development is altered in perinatal white matter injury. Nat. Neurosci. 18, 674–682 (2015).
Shiotsuki, H. et al. A rotarod test for evaluation of motor skill learning. J. Neurosci. Methods 189, 180–185 (2010).
Li, W., Ma, L., Yang, G. & Gan, W.-B. REM sleep selectively prunes and maintains new synapses in development and learning. Nat. Neurosci. 20, 427–437 (2017).
Yang, G. et al. Sleep promotes branch-specific formation of dendritic spines after learning. Science 344, 1173–1178 (2014).
Ma, L. et al. Experience-dependent plasticity of dendritic spines of layer 2/3 pyramidal neurons in the mouse cortex. Dev. Neurobiol. 76, 277–286 (2016).
Wang, L. et al. Modulation of social deficits and repetitive behaviors in a mouse model of autism: the role of the nicotinic cholinergic system. Psychopharmacology 232, 4303–4316 (2015).
Chen, C.-C., Gilmore, A. & Zuo, Y. Study motor skill learning by single-pellet reaching tasks in mice. J. Vis. Exp. https://doi.org/10.3791/51238 (2014).
Hodges, J. L. et al. Astrocytic contributions to synaptic and learning abnormalities in a mouse model of fragile X syndrome. Biol. Psychiatry 82, 139–149 (2017).
Acknowledgements
We thank A. Wafa Syed and S. Bhuvanendran for technical assistance. We also thank A. I. Son for critical reading of the manuscript. This work was supported by National Institutes of Health grants R01AA025215 (to K.H.-T.), R01AA026272 (to M.T. and K.H.-T.) and UH2AA026106 as part of the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (to K.H.-T. and M.T.). The study was also funded by the Scott-Gentle Foundation (to K.H.-T. and M.T.). This study was supported by award 1U54HD090257-01 from the the National Institutes of Health, District of Columbia Intellectual and Developmental Disabilities Research Center award program.
Author information
Authors and Affiliations
Contributions
M.T. and K.H.-T. designed the project. S.M., Y.I.K., A.S., L.W., S.I., P.L., T.S., A.B. and K.H.-T. performed experiments, S.M., S.J.P., Y.I.K., L.W., T.S., M.T. and K.H.-T. analyzed the data. S.M., S.J.P., Y.I.K., M.T. and K.H.-T. wrote the manuscript. Z.Q., F.I., H.N., K.I., J.G.C. and J.S.L. discussed the results and implications and commented on the manuscript. J.G.C. oversees the Intellectual and Developmental Disabilities Research Center-supported animal behavior core where the animal behavioral experiments were conducted.
Corresponding authors
Ethics declarations
Competing interests
K.H-T., M.T. and S.M. are the founders of Cogthera LLC, a biotechnology company with a mission to develop SK2 blockers for neurocognitive disorders.
Additional information
Peer review information Nature Neuroscience thanks Kelly Huffman and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 PAE mice show normal gross brain morphology, locomotor activity and anxiety-like behavior.
(a,b) There are no differences in the brain size/shape (a) or weight (b) between control and PAE mice. P = 0.75 by two-tailed Student’s t-test [n = 10 (5 males and 5 females) per group]. In the box plot, the line within the box indicates the median, and the upper and lower edges of the box represent the 25th and 75th percentiles, respectively. The upper and lower whisker boundaries indicate the 10th and 90th percentiles, respectively, and dots indicate outliers. (c-f) No abnormalities were observed in total distance (c), horizontal activity (d), vertical activity (e) or center time (f) in PAE mice in the open field test; F(1,38) = 0.07, P = 0.79 (c), F(1,38) = 0.004, P = 0.95 (d), F(1,38) = 0.44, P = 0.51 (e), F(1,38) = 0.21, P = 0.65 (f) by two-way repeated measures ANOVA (n = 20 animals per group). No significant differences in comparisons at each time point by two-tailed Student’s t-test. Graphs show mean ± SEM.
Extended Data Fig. 2 Learning impairment lasts over a period of 5 days in PAE mice.
a, The accelerated rotarod test was performed with the same daily schedule as shown in Fig. 1c for 5 days. PAE mice show significantly shorter latency to fall compared to control. F(1,19) = 28.3, P = 0.0001 by two-way repeated measures ANOVA. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 by Tukey’s test (control: n = 11 animals, PAE: n = 10 animals). Graph shows mean ± SEM. b, Learning index over the 5-day period is lower in PAE mice. *P = 0.015 by two-tailed Student’s t-test (control: n = 11 animals, PAE: n = 10 animals). c, The accelerated rotarod test was performed for 5 days with 3-month-old animals. No significant differences were observed in body weight between control and PAE mice; P = 0.243 by two-tailed Student’s t-test (control: n = 12 animals, PAE: n = 14 animals). d, PAE mice show significantly shorter latency to fall compared to control mice. F(1,24) = 11.57, P = 0.003 by two-way repeated measures ANOVA. *P < 0.05 by Tukey’s test (control: n = 12 animals, PAE: n = 14 animals). Graph shows mean ± SEM. e, Learning index over the 5-days period is also lower in PAE mice. *P = 0.026 by two-tailed Student’s t-test (control: n = 12 animals, PAE: n = 14 animals). In box plots (b, c, e), the line within the box indicates the median, and the upper and lower edges of the box represent the 25th and 75th percentiles, respectively. The upper and lower whisker boundaries indicate the 10th and 90th percentiles, respectively, and dots indicate outliers.
Extended Data Fig. 3 Pellet grasping is impaired in PAE mice.
a, PAE does not affect body weight change through the phases of the single pellet reaching test [food deprivation: day 1-3, acclimatization: day 4-5, training/shaping: day 6-8, and testing: day 9-16 (test day 1-8)]; F(1,33) = 0.89, P = 0.35 by two-way repeated measures ANOVA (vehicle: n = 20 animals, PAE: n = 15 animals). Graph shows mean ± SEM. (b-c) A significant effect of PAE is observed during the testing phase, on the failure to grasp (b); F(1,30) = 40.21, P < 0.0001 by two-way repeated measures ANOVA, *P < 0.05, **P < 0.01 by Tukey’s test, but not on the drop during retrieval (vehicle: n = 17 animals, PAE: n = 15 animals). Graph shows mean ± SEM. (c); F(1,30) = 0.87, P = 0.36 by two-way repeated measures ANOVA (vehicle: n = 17 animals, PAE: n = 15 animals). Graph shows mean ± SEM. d, PAE does not affect the number of attempts per minute; F(1,30) = 3.08, P = 0.09 by two-way repeated measures ANOVA (vehicle: n = 17 animals, PAE: n = 15 animals). Graph shows mean ± SEM.
Extended Data Fig. 4 Normal radial positioning and morphology of layer III neurons in M1 in PAE mice at P30.
a, The percentage of reporter− (control and PAE) and reporter+ (PAE) neurons among electroporated neurons in equally divided bins 1 to 10 spanning the upper to lower part of the entire thickness of the cerebral cortex. No significant differences are found between the distribution patterns in each group; P < 0.2 by one-tailed Kolmogorov-Smirnov test (n=3 per group). Graph shows mean ± SEM. b, Representative tracing of the neuronal morphology in each group. (c-e) There are no significant morphological differences in reporter+ cortical neurons in PAE mice compared with reporter− neurons in both control and PAE mice; Number of dendrites/neuron c, F(2,122) = 1.46, P = 0.24 (total), F(2,122) = 1.47, P = 0.23 (apical), F(2,122) = 0.84, P = 0.43 (basal); number of branches/neuron [reporter− (control, PAE): n = 40 cells each, reporter+ (PAE): n = 45 cells] d, F(2,122) = 0.17, P = 0.84 (apical primary), F(2,122) = 0.31, P = 0.73 (apical secondary), F(2,122) = 0.86, P = 0.43 (apical tertiary), F(2,122) = 0.21, P = 0.81 (basal primary), F(2,122) = 2.19, P = 0.12 (basal secondary), F(2,122) = 1.13, P = 0.33 (basal tertiary); length of dendrites/neuron [reporter− (control, PAE): n = 40 cells each, reporter+ (PAE): n = 45 cells] e, F(2,22) = 1.02, P = 0.38 (apical primary), F(2,22) = 0.26, P = 0.77 (apical secondary), F(2,22) = 0.20, P = 0.820 (apical tertiary), F(2,22) = 0.01, P = 0.99 (basal primary), F(2,22) = 0.79, P = 0.47 (basal secondary), F(2,22) = 0.29, P = 0.08 (basal tertiary), all by one-way ANOVA [reporter− (control): n = 9 cells, reporter− (PAE): n = 8 cells, reporter+ (PAE): n = 8 cells]. In box plots (c-e), the line within the box indicates the median, and the upper and lower edges of the box represent the 25th and 75th percentiles, respectively. The upper and lower whisker boundaries indicate the 10th and 90th percentiles, respectively, and dots indicate outliers.
Extended Data Fig. 5 GO networks of the Blue and Green modules that are specific to reporter+ neurons.
GO networks enriched in the Blue (a) and Green (b) modules show unique GOs for each module.
Extended Data Fig. 6 Kcnn2 expression is not increased in other major brain regions involved in motor learning.
a, Immunohistochemistry for Kcnn2 (red) with nuclear staining with DAPI (blue) in the indicated brain regions in control and PAE mice at P30. Arrowheads indicate Kcnn2+ cells. (b-e) Quantification of Kcnn2+ cells in dorsal striatum (b), hippocampus (granule cell layer of dentate gyrus) (c), cerebellum (granular layer in lobule VI) (d) and layer V in M1 (e). P = 0.51 (b), 0.26 (c), 0.37 (d), and 0.002 (e) by two-tailed Student’s t-test (n = 10 per group). In box plots (b-e), the line within the box indicates the median, and the upper and lower edges of the box represent the 25th and 75th percentiles, respectively. The upper and lower whisker boundaries indicate the 10th and 90th percentiles, respectively, and dots indicate outliers.
Extended Data Fig. 7 Tamapin binding colocalizes with Kcnn2 protein.
Biotinylated Tamapin (or vehicle-only control) was injected i.p. to control and PAE mice at P30. The cortex was fixed 30 minutes later for staining of biotin (green) and Kcnn2 (red). (a) Labeling for biotin (arrowhead) in layer III in M1 shows the co-localization of Tamapin and Kcnn2 protein. (b) Many Kcnn2+ cells in layer III in M1 are co-labeled for biotin in biotinylated Tamapin-injected PAE mice; ***P < 0.0001 by two-tailed Mann-Whitney U test (n = 10 per group). In the box plot, the line within the box indicates the median, and the upper and lower edges of the box represent the 25th and 75th percentiles, respectively. The upper and lower whisker boundaries indicate the 10th and 90th percentiles, respectively, and dots indicate outliers.
Extended Data Fig. 8 Minimal binding of Tamapin to Kcnn1+ or Kcnn3+ cells.
a, Biotinylated Tamapin was injected i.p. to PAE mice at P30. The cortex was fixed 30 minutes later for staining of biotin (green) and Kcnn1, 2, or 3 (red) in the hilus, in which Kcnn1, 2, and 3 have distinct expression patterns. Insets show higher magnification views of the areas outlined by broken lines. b, The percentage of biotin-labeled cells among the cells that express Kcnn1, 2, or 3 in the hilus, showing specific binding of biotinylated Tamapin to Kcnn2+ cells. F(2,21) = 148.21, P < 0.0001 by one-way ANOVA, **P < 0.01 by Tukey test (n = 8 brains per group). In the box plot, the line within the box indicates the median, and the upper and lower edges of the box represent the 25th and 75th percentiles, respectively. The upper and lower whisker boundaries indicate the 10th and 90th percentiles, respectively, and dots indicate outliers.
Extended Data Fig. 9 Tamapin does not alter locomotor activity or anxiety-like behavior.
(a-h) The open field test shows that locomotor activity, measured by total distance (a, e), horizontal activity (b, f) and vertical activity (c, g), as well as anxiety-like behavior, measured by center time (d, h), are not altered by postnatal Tamapin administration in both control (a-d) and PAE (e-h) mice; F(1,18) = 0.06, P = 0.81 (a), F(1,18) = 0.03, P = 0.87 (b), F(1,16) = 0.88, P = 0.36 (c), F(1,18) = 0.01, P = 0.92 (d), F(1,18) = 0.001, P = 1.00 (e), F(1,18) = 0.06, P = 0.81 (f), F(1,17) = 0.22, P = 0.65 (g), F(1,18) = 0.21, P = 0.65 (h) by two-way repeated measures ANOVA (n = 10 animals per group). Separate sets of mice were used for open field testing. Graph shows mean ± SEM.
Extended Data Fig. 10 Knockdown of Kcnn2 in layer II/III neurons in M1 improves motor learning deficits in PAE mice.
a, Timeline of the experiment. b, Representative image of a brain that received Kcnn2 (or control) knockdown (visualized by co-expressed GFP) in the motor area (outlined by red rectangles). c, Immunohistochemistry for Kcnn2 (red) shows that Kcnn2 shRNA, but not control shRNA, suppresses the increase in Kcnn2 expression in layer III neurons in M1 in PAE mice. Arrowheads indicate Kcnn2+ cells among GFP+ electroporated cells. d, Percentage of Kcnn2+ cells among GFP+ electroporated cells in the indicated experimental groups. **P = 0.001 by two-tailed Student’s t-test (n = 10 per group). (e, f) Motor learning deficits in PAE mice, revealed by lower success rate (e) and learning index (f) in the single pellet reaching test, are mitigated by Kcnn2 knockdown in layer II/III neurons in M1. A significant interaction was observed between the effects of condition (treatment plus shRNA) and trial (e); F(1,11) = 2.80, P = 0.01 by two-way repeated measures ANOVA, *P < 0.05, **P < 0.01 by simple main effect test [PAE (Kcnn2 shRNA−) vs PAE (Kcnn2 shRNA+)], and between treatment and shRNA (f); F(1,24) = 5.55, P = 0.03 by two-way ANOVA, **P < 0.05, ***P < 0.005 by simple main effect test (n = 10 animals per group). Graph shows mean ± SEM. In box plots (d, f), the line within the box indicates the median, and the upper and lower edges of the box represent the 25th and 75th percentiles, respectively. The upper and lower whisker boundaries indicate the 10th and 90th percentiles, respectively, and dots indicate outliers.
Supplementary information
Supplementary Information
Supplementary Figs 1–13.
Supplementary Tables
Supplementary Table 1. List of the entire gene sets detected by single-cell RNA-sequencing. Supplementary Table 2. ROKU ranking of single-cell RNA-sequencing data of reporter+ neurons. Ranking of gene expression specificity in reporter+ cell population in PAE mice. Supplementary Table 3. List of the genes in Fig. 2h and i. Supplementary Table 4. List of the genes classified into gene modules defined by WGCNA. Supplementary Table 5. Reporter+ cell-specific genes defined by both WGCNA and ROKU. Supplementary Table 6. ROKU ranking of single-cell RNA-sequencing data of reporter− neurons in PAE mice. Ranking of gene expression specificity in reporter− cell population in PAE mice. Supplementary Table 7. ROKU ranking of single-cell RNA-sequencing data of reporter− neurons in control mice. Ranking of gene expression specificity in reporter- cell population in control (PBS-exposed) mice.
Rights and permissions
About this article
Cite this article
Mohammad, S., Page, S.J., Wang, L. et al. Kcnn2 blockade reverses learning deficits in a mouse model of fetal alcohol spectrum disorders. Nat Neurosci 23, 533–543 (2020). https://doi.org/10.1038/s41593-020-0592-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-020-0592-z
This article is cited by
-
Fatty acid metabolism changes in association with neurobehavioral deficits in animal models of fetal alcohol spectrum disorders
Communications Biology (2023)
-
Gestational ethanol exposure impairs motor skills in female mice through dysregulated striatal dopamine and acetylcholine function
Neuropsychopharmacology (2023)
-
Fetal alcohol spectrum disorders
Nature Reviews Disease Primers (2023)
-
Activation of the anterior cingulate cortex ameliorates anxiety in a preclinical model of fetal alcohol spectrum disorders
Translational Psychiatry (2022)
-
Long-term spatial tracking of cells affected by environmental insults
Journal of Neurodevelopmental Disorders (2020)