Abstract
Prolonged obesity is associated with cerebrovascular dysfunction; however, the underlying mechanisms remain largely unclear. In the present study, using a prolonged obesity mouse model that suffers from basilar artery (BA) abnormalities, we find that microglial transforming growth factor β-activated kinase 1 (Tak1) is over-activated in the brainstem. Both pharmacological inhibition primarily in the brainstem and genetic microglia-selective deletion of Tak1 ameliorated BA vascular dysfunction. Conversely, microglia-specific activation of Tak1 in the brainstem was sufficient to cause an impairment in BA function in chow-fed mice. Mechanistically, Tak1 activation leads to increased interleukin-18 (IL-18) production, whereas blockade of IL-18 receptor in the brain helped protect against cerebrovascular dysfunction despite prolonged obesity. Microglia-selective deletion of Tak1 also protects against ischemic stroke in prolonged obesity. Taken together, these findings provide evidence that microglial Tak1 in the brain, and particularly the brainstem, contributes to the pathogenesis of obesity-associated cerebrovascular dysfunction.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
References
Kotsis, V. T., Stabouli, S. V., Papamichael, C. M. & Zakopoulos, N. A. Impact of obesity in intima media thickness of carotid arteries. Obesity 14, 1708–1715 (2006).
Grassi, G. et al. Structural and functional alterations of subcutaneous small resistance arteries in severe human obesity. Obesity 18, 92–98 (2010).
Chatterjee, S. et al. Inhibition of glycosphingolipid synthesis ameliorates atherosclerosis and arterial stiffness in apolipoprotein E−/− mice and rabbits fed a high-fat and -cholesterol diet. Circulation 129, 2403–2413 (2014).
Candela, J., Velmurugan, G. V. & White, C. Hydrogen sulfide depletion contributes to microvascular remodeling in obesity. Am. J. Physiol. Heart Circ. Physiol. 310, H1071–1080 (2016).
Tounian, P. et al. Presence of increased stiffness of the common carotid artery and endothelial dysfunction in severely obese children: a prospective study. Lancet 358, 1400–1404 (2001).
Schafer, N. et al. Endothelial mineralocorticoid receptor activation mediates endothelial dysfunction in diet-induced obesity. Eur. Heart J. 34, 3515–3524 (2013).
Ungvari, Z. et al. Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nat. Rev. Cardiol. 15, 555–565 (2018).
Endemann, D. H. & Schiffrin, E. L. Endothelial dysfunction. J. Am. Soc. Nephrol. 15, 1983–1992 (2004).
Gimbrone, M. A. Jr. & Garcia-Cardena, G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ. Res. 118, 620–636 (2016).
Tsuchiya, K. et al. FoxOs integrate pleiotropic actions of insulin in vascular endothelium to protect mice from atherosclerosis. Cell Metab. 15, 372–381 (2012).
Vanhoutte, P. M., Shimokawa, H., Feletou, M. & Tang, E. H. Endothelial dysfunction and vascular disease - a 30th anniversary update. Acta Physiol. 219, 22–96 (2017).
de Boer, R. A. et al. Association of cardiovascular biomarkers with incident heart failure with preserved and reduced ejection fraction. JAMA Cardiol. 3, 215–224 (2018).
Heitzer, T., Schlinzig, T., Krohn, K., Meinertz, T. & Munzel, T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation 104, 2673–2678 (2001).
O’Leary, D. H. & Bots, M. L. Imaging of atherosclerosis: carotid intima-media thickness. Eur. Heart J. 31, 1682–1689 (2010).
Polak, J. F. et al. Carotid-wall intima-media thickness and cardiovascular events. N. Engl. J. Med 365, 213–221 (2011).
Hotamisligil, G. S. Inflammation, metaflammation and immunometabolic disorders. Nature 542, 177–185 (2017).
Lee, Y. S., Wollam, J. & Olefsky, J. M. An integrated view of immunometabolism. Cell 172, 22–40 (2018).
Brestoff, J. R. & Artis, D. Immune regulation of metabolic homeostasis in health and disease. Cell 161, 146–160 (2015).
Cai, D. S. Neuroinflammation and neurodegeneration in overnutrition-induced diseases. Trends Endocrin. Met 24, 40–47 (2013).
Kalin, S. et al. Hypothalamic innate immune reaction in obesity. Nat. Rev. Endocrinol. 11, 339–351 (2015).
Horvath, T. L. et al. Synaptic input organization of the melanocortin system predicts diet-induced hypothalamic reactive gliosis and obesity. Proc. Natl Acad. Sci. USA 107, 14875–14880 (2010).
Zhang, Y. L., Reichel, J. M., Han, C., Zuniga-Hertz, J. P. & Cai, D. S. Astrocytic process plasticity and IKKβ/NF-κB in central control of blood glucose, blood pressure, and body weight. Cell Metab. 25, 1091–1102 (2017).
Valdearcos, M. et al. Microglial inflammatory signaling orchestrates the hypothalamic immune response to dietary excess and mediates obesity susceptibility. Cell Metab. 26, 185–197 (2017).
Thaler, J. P. et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Invest. 122, 153–162 (2012).
Baufeld, C., Osterloh, A., Prokop, S., Miller, K. R. & Heppner, F. L. High-fat diet-induced brain region-specific phenotypic spectrum of CNS resident microglia. Acta Neuropathol. 132, 361–375 (2016).
Schur, E. A. et al. Radiologic evidence that hypothalamic gliosis is associated with obesity and insulin resistance in humans. Obesity 23, 2142–2148 (2015).
Yan, J. et al. Obesity- and aging-induced excess of central transforming growth factor-β potentiates diabetic development via an RNA stress response. Nat. Med. 20, 1001–1008 (2014).
Sato, S. et al. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat. Immunol. 6, 1087–1095 (2005).
Wan, Y. Y., Chi, H., Xie, M., Schneider, M. D. & Flavell, R. A. The kinase TAK1 integrates antigen and cytokine receptor signaling for T cell development, survival and function. Nat. Immunol. 7, 851–858 (2006).
Mulvany, M. J. & Halpern, W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ. Res. 41, 19–26 (1977).
Spiers, A. & Padmanabhan, N. A guide to wire myography. Methods Mol. Med. 108, 91–104 (2005).
Ninomiya-Tsuji, J. et al. A resorcylic acid lactone, 5Z-7-oxozeaenol, prevents inflammation by inhibiting the catalytic activity of TAK1 MAPK kinase kinase. J. Biol. Chem. 278, 18485–18490 (2003).
Xie, M. et al. A pivotal role for endogenous TGF-β-activated kinase-1 in the LKB1/AMP-activated protein kinase energy-sensor pathway. Proc. Natl Acad. Sci. USA 103, 17378–17383 (2006).
Yona, S. et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38, 79–91 (2013).
Yamaguchi, K. et al. Identification of a member of the MAPKKK family as a potential mediator of TGF-β signal transduction. Science 270, 2008–2011 (1995).
Zhang, G. et al. Hypothalamic programming of systemic ageing involving IKK-β, NF-κB and GnRH. Nature 497, 211–216 (2013).
Hanisch, U. K. Microglia as a source and target of cytokines. Glia 40, 140–155 (2002).
Kernan, W. N., Inzucchi, S. E., Sawan, C., Macko, R. F. & Furie, K. L. Obesity: a stubbornly obvious target for stroke prevention. Stroke 44, 278–286 (2013).
Bazzano, L. A. et al. Body mass index and risk of stroke among Chinese men and women. Ann. Neurol. 67, 11–20 (2010).
Zhou, L. et al. Treatment of cerebral ischemia by disrupting ischemia-induced interaction of nNOS with PSD-95. Nat. Med 16, 1439–1443 (2010).
Lackey, D. E. & Olefsky, J. M. Regulation of metabolism by the innate immune system. Nat. Rev. Endocrinol. 12, 15–28 (2016).
Zhang, X. Q. et al. Hypothalamic IKKβ/NF-κB and ER stress link overnutrition to energy imbalance and obesity. Cell 135, 61–73 (2008).
Kleinridders, A. et al. MyD88 signaling in the CNS is required for development of fatty acid-induced leptin resistance and diet-induced obesity. Cell Metab. 10, 249–259 (2009).
Gao, Y. Q. et al. Dietary sugars, not lipids, drive hypothalamic inflammation. Mol. Metab. 6, 897–908 (2017).
Yi, C. X. et al. TNFα drives mitochondrial stress in POMC neurons in obesity. Nat. Commun. 8, 15143 (2017).
Qian, L. et al. β2-Adrenergic receptor activation prevents rodent dopaminergic neurotoxicity by inhibiting microglia via a novel signaling pathway. J. Immunol. 186, 4443–4454 (2011).
Goldmann, T. et al. A new type of microglia gene targeting shows TAK1 to be pivotal in CNS autoimmune inflammation. Nat. Neurosci. 16, 1618–1626 (2013).
Masuda, T. et al. Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature 566, 388–392 (2019).
Gerdes, N. et al. Expression of interleukin (IL)-18 and functional IL-18 receptor on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for atherogenesis. J. Exp. Med 195, 245–257 (2002).
Fix, C., Bingham, K. & Carver, W. Effects of interleukin-18 on cardiac fibroblast function and gene expression. Cytokine 53, 19–28 (2011).
Li, M. J. et al. Optimal promoter usage for lentiviral vector-mediated transduction of cultured central nervous system cells. J. Neurosci. Meth. 189, 56–64 (2010).
Hickstein, D. D., Baker, D. M., Gollahon, K. A. & Back, A. L. Identification of the promoter of the myelomonocytic leukocyte integrin CD11b. Proc. Natl Acad. Sci. USA 89, 2105–2109 (1992).
Wu, L. et al. Caffeine inhibits hypothalamic A1R to excite oxytocin neuron and ameliorate dietary obesity in mice. Nat. Commun. 8, 15904 (2017).
Purkayastha, S., Zhang, G. & Cai, D. S. Uncoupling the mechanisms of obesity and hypertension by targeting hypothalamic IKK-β and NF-κB. Nat. Med. 17, 883–887 (2011).
Zhao, Y. et al. The deubiquitinase CYLD targets Smad7 protein to regulate transforming growth factor β (TGF-β) signaling and the development of regulatory T cells. J. Biol. Chem. 286, 40520–40530 (2011).
Lee, S. et al. Autocrine VEGF signaling is required for vascular homeostasis. Cell 130, 691–703 (2007).
Kim, M. S. et al. Rapid linkage of innate immunological signals to adaptive immunity by the brain–fat axis. Nat. Immunol. 16, 525–533 (2015).
Acknowledgements
We thank M. Li (Washington University in St. Louis) for sharing the LV-GFAP-GFP plasmid. The present study was supported by the National Natural Science Foundation of China (grant no. 91539125, 81573146), the Junior Thousand Talents Program of China and the Huazhong University of Science and Technology startup fund (all to G.Z.). J.L. was supported by the National Natural Science Foundation of China (grant no. 81570774) and the National Key Research and Development Program (grant no. 2018YFC1003504).
Author information
Authors and Affiliations
Contributions
Q.S. designed the study, performed the experiments, analyzed the data and drafted the manuscript. Z.C., F.Z., S.P., T.Z., X.C., L.Z., S.Z. and J.Q. performed the experiments and analyzed the data. J.L. designed the experiments and analyzed the data. D.C. co-designed the study and co-wrote the paper. G.Z. conceived the study, designed the experiments, analyzed the data and co-wrote the paper. All authors commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Characterization of the basilar artery in DIO mice.
a-c, Body weight (a), fat mass (b) and lean mass (c) of C57 BL/6 mice fed a normal chow or a high-fat diet (HFD) for 32 weeks. *P = 0.000001, t(14) = 8.52 (a) or P = 0.00001, t(14) = 6.75 (b). n = 8 mice per group. d, Plasma total cholesterol (TC) levels. *P = 0.002, t(14) = 3.91. n = 8 mice per group. e, The internal perimeter of basilar artery. n = 5 mice per group. f, Representative images of Verhoeff’s Van Gieson elastic (EVG) staining of basilar artery. Elastic fiber stains blue-black. Scale bars, 20 μm. n = 7 (Chow) or 6 (HFD) mice. g, The relative level of elastin in basilar artery. au, arbitrary unit. n = 7 (Chow) or 6 (HFD) mice. h, Male C57 BL/6 mice (6 weeks old) were fed a chow or HFD for 12 weeks. Immunofluorescent staining for α-smooth muscle actin (α-SMA, green), a protein marker for smooth muscle cells, was performed. Representative images show the immunostaining of basilar artery. Cell nuclei were counterstained with DAPI (blue). Scale bars, 20 µm. n = 3 mice per group. i, Relative content of α-SMA in the wall of basilar artery. au, arbitrary unit. n = 3 mice per group. j, Immunofluorescent staining for Vimentin (green), a protein marker for fibroblasts, was performed. Representative images show the immunostaining of basilar artery. Cell nuclei were counterstained with DAPI (blue). Scale bars, 20 µm. n = 3 mice per group. k, Relative content of Vimentin in the wall of basilar artery. au, arbitrary unit. n = 3 mice per group. l, The dose-response curves to sodium nitroprusside (SNP). n = 7 (Chow) or 8 (HFD) mice. Data are presented as mean ± SEM. Two-tailed Student’s t-test was used for statistical analysis (a, b, d).
Extended Data Fig. 2 Expression and activity of Tak1 in the neurons of brainstem.
a, Double immunofluorescence staining for Tak1 (red) and Hu C/D (a neuronal marker, green). The region of brainstem close to basilar artery is shown. Arrows indicate double positive cells. Scale bars, 20 µm. n = 4 mice per group. b, Male C57 BL/6 mice (6 weeks old) were fed a chow or a HFD for 12 weeks, and then double immunofluorescence staining for pTak1 (red) and Hu C/D (green) was performed. The region of brainstem adjacent to the basilar artery is shown. Scale bars, 20 µm. n = 4 mice per group. c, Density of neuron in the brainstem. n = 4 mice per group. d, Percentage of neurons that were pTak1+ in the brainstem. n = 4 mice per group. Data are presented as mean ± SEM.
Extended Data Fig. 3 Vascular dysfunction of basilar artery in chow-fed ob/ob mice.
a, Body weight of male wt/wt and ob/ob mice (12 weeks old) fed a chow diet. ***P = 0.00000001, t(8) = 23.62. n = 5 mice per group. b, Representative images of H&E, Masson’s trichrome and EVG stainings of basilar artery. Collagen stains blue in Masson’s trichrome staining. Elastic fiber stains blue-black in EVG staining. Scale bars, 20 μm. For H&E or EVG staining, n = 4 (wt/wt) or 5 (ob/ob) mice. For Masson’s trichrome staining, n = 4 mice per group. c, The wall thickness of basilar artery. *P = 0.00001, t(7) = 11.13. n = 4 (wt/wt) or 5 (ob/ob) mice. d, Percentage of collagen (blue) area over wall area in the basilar artery. *P = 0.001, t(6) = 6.01. n = 4 mice per group. e, The relative elastin level in basilar artery. au, arbitrary unit. n = 4 (wt/wt) or 5 (ob/ob) mice. f, Immunofluorescent staining for p-eNOS (green) in the basilar artery. Scale bars, 50 μm. n = 4 mice per group. g, Relative p-eNOS level in the basilar artery. au, arbitrary unit. *P = 0.0003, t(6) = 7.66. n = 4 mice per group. h, The basilar artery of ob/ob mice is less sensitive to Ach in wire myography. F(1, 40) = 26.8 for column factor. F(4, 40) = 8.92 for row factor. *P = 0.020, logM (Ach) = -6; P = 0.023, logM (Ach) = -5 or P = 0.012, logM (Ach) = -4. n = 5 mice per group. i, Double immunofluorescence staining for pTak1 (red) and Iba1 (green) in the brainstem of wt/wt and ob/ob mice. Scale bars, 20 µm. n = 4 mice per group. (j) Percentage of pTak1+ microglia in the brainstem. *P = 0.0002, t(6) = 8.35. n = 4 mice per group. Data are presented as mean ± SEM. Two-tailed Student’s t-test (a, c, d, g, j) and two-way ANOVA with Bonferroni’s post hoc test (h) were used for statistical analysis.
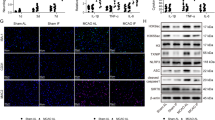
Extended Data Fig. 4 Expression of Tak1 in the BA and perivascular macrophages in Cx3cr1CreER, Tak1Loxp/Loxp mice.
a, Male C57 BL/6 mice (12 weeks old) were perfused and then the brains were sectioned. Double immunofluorescence staining for Tak1 (red) and CD31, α-SMA or Vimentin (green) of basilar artery was performed. Cell nuclei were counterstained with DAPI (blue). Scale bars, 10 µm. n = 3 mice per group. b, Adult male Cx3cr1CreER, Tak1Loxp/Loxp and Cx3Cr1CreER, Tak1Loxp/Loxp (KO) mice were anesthetized and perfused with 4% PFA. Double immunofluorescence staining for Tak1 (red) and CD31 (green) of basilar artery was performed. Cell nuclei were counterstained with DAPI (blue). Scale bars, 10 µm. n = 3 mice per group. c, Double immunofluorescence staining for Tak1 (red) and CD11b (green, a macrophage marker). Arrows indicate double positive cells. Cell nuclei were counterstained with DAPI (blue). Scale bars, 10 µm. n = 3 mice per group.
Extended Data Fig. 5 Effect of microglial Tak1 on energy balance, and the internal perimeter or elastin content in basilar artery.
a, Male Cx3cr1CreER, Tak1Loxp/Loxp and Cx3cr1CreER, Tak1Loxp/Loxp (KO) mice were fed a chow or high-fat diet (HFD) starting at 6 weeks of age. Body weight was then monitored. F(5, 196) = 31.6 for column factor. F(6, 196) = 27.7 for row factor. *P < 0.05 (HFD versus Chow). **P < 0.01 (HFD versus Chow). n = 5 (Chow), 6 (Cx3cr1CreER, HFD), 7 (Tak1Loxp/Loxp, HFD) or 6 (KO, HFD) mice per group. b,c, Fat mass (b) and lean mass (c) immediately before HFD treatment. n = 5 mice per group. d,e, Fat mass (d) and lean mass (e) after 24 weeks of chow or HFD treatment. In d, F(5, 38) = 6.52. *P < 0.05. n = 6 (Chow), 8 (Cx3cr1CreER, HFD), 9 (Tak1Loxp/Loxp, HFD) or 9 (KO, HFD) mice per group. (f) Cumulative food intake. n = 6 (Cx3cr1CreER, Chow), 7 (Tak1Loxp/Loxp, Chow), 6 (KO, Chow), 8 (Cx3cr1CreER, HFD), 10 (Tak1Loxp/Loxp, HFD) or 10 (KO, HFD) mice. g, Internal perimeter of basilar artery. n = 4 (Cx3cr1CreER, Chow), 3 (Tak1Loxp/Loxp, Chow), 4 (KO, Chow), 3 (Cx3cr1CreER, HFD), 3 (Tak1Loxp/Loxp, HFD) or 4 (KO, HFD) mice. h, EVG staining of basilar artery. Blue-black color represents elastin fibers. Scale bars, 20 μm. n = 5 (Cx3cr1CreER, Chow), 6 (Tak1Loxp/Loxp, Chow), 4 (KO, Chow), 6 (Cx3cr1CreER, HFD), 5 (Tak1Loxp/Loxp, HFD) or 5 (KO, HFD) mice. i, Relative elastin level in basilar artery. au, arbitrary unit. n = 5 (Cx3cr1CreER, Chow), 6 (Tak1Loxp/Loxp, Chow), 4 (KO, Chow), 6 (Cx3cr1CreER, HFD), 5 (Tak1Loxp/Loxp, HFD) or 5 (KO, HFD) mice. Data are presented as mean ± SEM. One-way (d) or two-way (a) ANOVA with Bonferroni’s post hoc test.
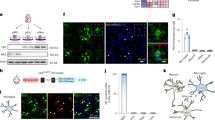
Extended Data Fig. 6 Effect of Tak1 activation in the microglia of brainstem on energy balance.
a, Schematics of the CD11b-Ctrl-Lenti and CD11b-Tak1CA-Lenti plasmids. HA, Hemagglutinin epitope tag. Tak1CA, constitutively active Tak1. 2A, 2A peptide. EGFP, Enhanced green fluorescent protein. LTR, Long terminal repeat. b, Representative images showing the expression of EGFP in the brainstem region close to basilar artery after the injection of CD11b-Ctrl-Lenti or CD11b-Tak1CA-Lenti (CD11b-Tak1CA-L) viruses. Cell nuclei were counterstained with DAPI (blue). Scale bars, 20 µm. n = 3 mice per group. c, Adult male mice were injected CD11b-Ctrl-Lenti viruses into the bottom region of brainstem. A week later, mice were perfused with saline and then the brainstem was dissected out. After tissue digestion, cells were isolated by using the Percoll gradients, blocked by FcX, and then labelled with PE-conjugated anti-mouse Cx3cr1 antibody. A representative flow cytometry plot is shown. n = 6 mice per group. d,e, Male mice (12 weeks old) were injected CD11b-Ctrl-Lenti or CD11b-Tak1CA-Lenti viruses into the brainstem region close to basilar artery. Body weight (d) and cumulative food intake (e) were then assessed. n = 8 (d) or 10 (e) mice per group. Data are presented as mean ± SEM.
Extended Data Fig. 7 Effects of Tak1 activation in the microglia of brainstem on BA morphology and function.
a, H&E and Masson’s trichrome stainings of basilar artery. Ctrl-Lenti, CD11b-Ctrl-Lenti. Tak1CA-Lenti, CD11b-Tak1CA-Lenti. Scale bars, 20 µm. For H&E staining, n = 5 mice per group. For Masson’s trichrome staining, n = 4 (Ctrl-Lenti) or 3 (Tak1CA-Lenti) mice. b, Thickness of basilar arterial wall. Ctrl-L, CD11b-Ctrl-Lenti. Tak1CA-L, CD11b-Tak1CA-Lenti. *P = 0.024, t(8) = 2.77. n = 5 mice per group. c, Percentage of collagen area over vessel wall area. *P = 0.01, t(5) = 4.0. n = 4 (Ctrl-L) or 3 (Tak1CA-L) mice. d, Immunofluorescent staining for p-eNOS (green) in the basilar artery. Scale bars, 50 µm. n = 4 mice per group. e, Relative p-eNOS fluorescence level in the basilar artery. au, arbitrary unit. *P = 0.002, t(6) = 5.14. n = 4 mice per group. f, C57 BL/6 mice (12 weeks old) were injected the indicated lentiviruses into brainstem. Four weeks later, basilar arteries were collected and lysed. The lysates were immunoblotted with p-eNOS and eNOS antibodies. Gapdh was used as a loading control. To prepare each sample, tissues from 8 mice were combined. g, Impairment of Ach-evoked relaxation of basilar artery of CD11b-Tak1CA-Lenti virus-injected mice. Ctrl-Lenti, CD11b-Ctrl-Lenti. F(1, 100) = 51.03 for column factor. F(4, 100) = 88.33 for row factor. *P = 0.002, logM (Ach) = -6; P = 0.001, logM (Ach) = -5; or P = 0.0008, logM (Ach) = -4. n = 11 mice per group. Uncropped western blots can be found in Source Data Extended Data Fig. 7. Data are presented as mean ± SEM. Two-tailed Student’s t-test (b, c, e) and two-way ANOVA with Bonferroni’s post hoc test (g) were used for statistical analysis.
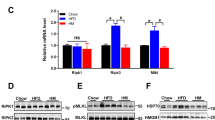
Extended Data Fig. 8 IL-18 suppresses eNOS activity in RAEC cells.
a, Quantitative RT-PCR analysis of the mRNA levels of cytokines and chemokines in the brainstem of chow- or HFD-fed mice. au, arbitrary unit. n = 6 (IL-1β, HFD or IL-23α, Chow) or 7 (all other groups) mice per group. b, Immunofluorescent staining for iNOS (red) and Iba1 (green). Tissue sections were counterstained with DAPI (blue) to visualize cell nuclei. Brainstem region close to basilar artery is shown. Scale bars, 20 µm. n = 3 mice per group. c, Percentage of iNOS+ microglia in chow- or HFD-fed mice. n = 3 mice per group. d, Rat aortic endothelial cells (RAECs) were incubated with vehicle (Ctrl) or IL-18 (20 ng ml-1) for 4 hrs. Total proteins were prepared and subjected to western blot analysis. β-Actin was used as a loading control. n = 3 cell cultures per group. e, RAECs were transfected with scramble RNA (Ctrl) or siRNA targeting IL-18Rα. The cells were further cultured for 4 days and then harvested for protein preparation. The proteins were subjected to western blot analysis. β-Actin was used as a loading control. n = 3 cell cultures per group. f, Quantification of the immunoblots shown in d and e. For p-eNOS/eNOS, *P = 0.005, t(4) = 5.55, Ctrl versus IL-18; or P = 0.03, t(4) = 3.3, Ctrl versus IL-18Rα siRNA. For IL-18Rα/β-Actin, *P = 0.0002, t(4) = 13.95. n = 3 cell cultures per group. Uncropped western blots can be found in Source Data Extended Data Fig. 8. Data are presented as mean ± SEM. Two-tailed Student’s t-test was used for statistical analysis (f).
Extended Data Fig. 9 Validation of pTak1 Thr 187 antibody.
a, HEK293T cells were transfected with vector, Tak1 wild type (WT) or Tak1 T187A plasmids for 48 h. Both the Tak1 WT and Tak1 T187A cDNAs were Flag-epitope tagged at the COOH-terminus. Cells were then serum-starved overnight, stimulated with vehicle or TGFβ1 (10 ng ml-1, 15 mins) and fixed with 4% PFA. Double immunofluorescence staining for pTak1 Thr 187 (red) and Flag (green) was performed. Cell nuclei were counterstained with DAPI (blue). Scale bars, 20 µm. Data shown are a representative of 3 independent experiments. b, HEK293T cells were transfected with the indicated plasmids. After stimulated with TGFβ1, the cells were lysed. Lysates were immunoblotted with anti-pTak1 Thr 187 (pTak1) and anti-Flag antibodies, respectively. β-Actin was used as a loading control. A representative of 3 independent experiments is shown. c, Quantification of the immunoblots for pTak1. au, arbitrary unit. F(5, 12) = 15.5. *P = 0.0002 (Vector, Vehicle versus Tak1 WT, TGFβ1); P = 0.0003 (Vector, TGFβ1 versus Tak1 WT, TGFβ1); P = 0.0007 (Tak1 WT, Vehicle versus Tak1 WT, TGFβ1); P = 0.0003 (Tak1 WT, TGFβ1 versus Tak1 T187A, Vehicle); or P = 0.0002 (Tak1 WT, TGFβ1 versus Tak1 T187A, TGFβ1). n = 3 independent experiments. Uncropped western blots can be found in Source Data Extended Data Fig. 9. Data are shown as mean ± SEM. One-way ANOVA with Bonferroni’s post hoc test.
Extended Data Fig. 10 Validation of p-eNOS antibody.
a, RAECs were transfected with scramble RNA or eNOS siRNA for 48 h. Cells were then serum-starved, stimulated with vehicle or VEGF (100 ng ml-1, 30 mins) and lysed. Lysates were immunoblotted with anti-p-eNOS and anti-eNOS antibodies, respectively. β-Actin was used as a loading control. n = 3 cell cultures per group. b,c, Quantification of the western blots. au, arbitrary unit. In b, F(3, 8) = 263.5. *P = 0.0000001 (scramble RNA, Vehicle versus scramble RNA, VEGF); P = 00000008 (scramble RNA, VEGF versus eNOS siRNA, Vehicle); or P = 0.00000007 (scramble RNA, VEGF versus eNOS siRNA, VEGF). In c, F(3, 8) = 87.6. *P = 0.00003 (scramble RNA, Vehicle versus eNOS siRNA, Vehicle); P = 0.00004 (scramble RNA, Vehicle versus eNOS siRNA, VEGF); P = 0.00001 (scramble RNA, VEGF versus eNOS siRNA, Vehicle); or P = 0.00001 (scramble RNA, VEGF versus eNOS siRNA, VEGF). n = 3 cell cultures per group. d, Immunofluorescent staining for p-eNOS (green) in RAEC cells. Scale bars, 20 µm. A representative of 3 independent experiments is shown. e, A schematic representation displaying the impact of microglia Tak1 in obesity-related cerebrovascular dysfunction. Obesity results in microgliosis and Tak1 activation in the microglia of brainstem, which then increases the production and secretion of IL-18. IL-18 acts on endothelial cells to decrease the activity of eNOS and reduces the production of nitric oxide (NO), thereby resulting in endothelial dysfunction. Action of IL-18 on other cell types leads to wall thickening and collagen deposition in basilar artery. Vascular dysfunction worsens the outcome of ischemic stroke in DIO mice. Yellow, microglia; grey, neuron; blue, astrocytes; green, endothelial cells. Uncropped western blots can be found in Source Data Extended Data Fig. 10. Data are presented as mean ± SEM. One-way ANOVA with Bonferroni’s post hoc test is used for statistical analysis.
Supplementary information
Supplementary Information
Supplementary Fig. 1 and Supplementary Tables 1 and 2.
Source data
Source Data Fig. 1
Uncropped western blots.
Source Data Fig. 4
Uncropped western blots.
Source Data Extended Data Fig. 7
Uncropped western blots.
Source Data Extended Data Fig. 8
Uncropped western blots.
Source Data Extended Data Fig. 9
Uncropped western blots.
Source Data Extended Data Fig. 10
Uncropped western blots.
Rights and permissions
About this article
Cite this article
Shen, Q., Chen, Z., Zhao, F. et al. Reversal of prolonged obesity-associated cerebrovascular dysfunction by inhibiting microglial Tak1. Nat Neurosci 23, 832–841 (2020). https://doi.org/10.1038/s41593-020-0642-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-020-0642-6
This article is cited by
-
Noteworthy perspectives on microglia in neuropsychiatric disorders
Journal of Neuroinflammation (2023)
-
TRIM45 causes neuronal damage by aggravating microglia-mediated neuroinflammation upon cerebral ischemia and reperfusion injury
Experimental & Molecular Medicine (2022)
-
Maternal high-fat diet in mice induces cerebrovascular, microglial and long-term behavioural alterations in offspring
Communications Biology (2022)
-
TAK1 mediates neuronal pyroptosis in early brain injury after subarachnoid hemorrhage
Journal of Neuroinflammation (2021)
-
Hypothalamic long noncoding RNA AK044061 is involved in the development of dietary obesity in mice
International Journal of Obesity (2021)