Abstract
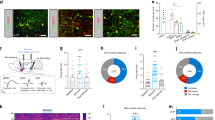
The ventral tegmental area (VTA) is important for reward processing and motivation. The anatomic organization of neurotransmitter-specific inputs to the VTA remains poorly resolved. In the present study, we mapped the major neurotransmitter projections to the VTA through cell-type-specific retrograde and anterograde tracing. We found that glutamatergic inputs arose from a variety of sources and displayed some connectivity biases toward specific VTA cell types. The sources of GABAergic projections were more widespread, displayed a high degree of differential innervation of subregions in the VTA and were largely biased toward synaptic contact with local GABA neurons. Inactivation of GABA release from the two major sources, locally derived versus distally derived, revealed distinct roles for these projections in behavioral regulation. Optogenetic manipulation of individual distal GABAergic inputs also revealed differential behavioral effects. These results demonstrate that GABAergic projections to the VTA are a major contributor to the regulation and diversification of the structure.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Datasets supporting the findings in the present study are available from the corresponding author upon reasonable request. All viral vectors used in this manuscript are available from the corresponding author upon reasonable request.
Code availability
Code used for behavioral analysis is available from the corresponding author upon reasonable request.
References
Morales, M. & Margolis, E. B. Ventral tegmental area: cellular heterogeneity, connectivity and behaviour. Nat. Rev. Neurosci. 18, 73–85 (2017).
Jhou, T. C., Fields, H. L., Baxter, M. G., Saper, C. B. & Holland, P. C. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron 61, 786–800 (2009).
Kaufling, J., Veinante, P., Pawlowski, S. A., Freund-Mercier, M. J. & Barrot, M. Afferents to the GABAergic tail of the ventral tegmental area in the rat. J. Compar. Neurol. 513, 597–621 (2009).
Brown, M. T. et al. Ventral tegmental area GABA projections pause accumbal cholinergic interneurons to enhance associative learning. Nature 492, 452–456 (2012).
Cohen, J. Y., Haesler, S., Vong, L., Lowell, B. B. & Uchida, N. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature 482, 85–88 (2012).
Yoo, J. H. et al. Ventral tegmental area glutamate neurons co-release GABA and promote positive reinforcement. Nat. Commun. 7, 13697 (2016).
Qi, J. et al. VTA glutamatergic inputs to nucleus accumbens drive aversion by acting on GABAergic interneurons. Nat. Neurosci. 19, 725–733 (2016).
Beier, K. T. et al. Circuit architecture of VTA dopamine neurons revealed by systematic input–output mapping. Cell 162, 622–634 (2015).
Lammel, S. et al. Input-specific control of reward and aversion in the ventral tegmental area. Nature 491, 212–217 (2012).
Menegas, W. et al. Dopamine neurons projecting to the posterior striatum form an anatomically distinct subclass. eLife 4, e10032 (2015).
Yang, H. et al. Nucleus accumbens subnuclei regulate motivated behavior via direct inhibition and disinhibition of VTA dopamine subpopulations. Neuron 97, 434–449 (2018). e434.
Watabe-Uchida, M., Zhu, L., Ogawa, S. K., Vamanrao, A. & Uchida, N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron 74, 858–873 (2012).
Faget, L. et al. Afferent inputs to neurotransmitter-defined cell types in the ventral tegmental area. Cell Rep. 15, 2796–2808 (2016).
Sesack, S. R. & Grace, A. A. Cortico-basal ganglia reward network: microcircuitry. Neuropsychopharmacology 35, 27–47 (2010).
Geisler, S., Derst, C., Veh, R. W. & Zahm, D. S. Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci. 27, 5730–5743 (2007).
Rinvik, E. & Grofova, I. Observations on the fine structure of the substantia nigra in the cat. Exp. Brain Res. 11, 229–248 (1970).
Chung, A. S., Miller, S. M., Sun, Y., Xu, X. & Zweifel, L. S. Sexual congruency in the connectome and translatome of VTA dopamine neurons. Scien. Rep. 7, 11120 (2017).
Gore, B. B. et al. Roundabout receptor 2 maintains inhibitory control of the adult midbrain. eLife 6, e23858 (2017).
Jennings, J. H. et al. Distinct extended amygdala circuits for divergent motivational states. Nature 496, 224–228 (2013).
Nieh, E. H. et al. Inhibitory input from the lateral hypothalamus to the ventral tegmental area disinhibits dopamine neurons and promotes behavioral activation. Neuron 90, 1286–1298 (2016).
McHenry, J. A. et al. Hormonal gain control of a medial preoptic area social reward circuit. Nat. Neurosci. 20, 449–458 (2017).
Hjelmstad, G. O., Xia, Y., Margolis, E. B. & Fields, H. L. Opioid modulation of ventral pallidal afferents to ventral tegmental area neurons. J. Neurosci. 33, 6454–6459 (2013).
Bocklisch, C. et al. Cocaine disinhibits dopamine neurons by potentiation of GABA transmission in the ventral tegmental area. Science 341, 1521–1525 (2013).
Sanford, C. A. et al. A central amygdala CRF crcuit facilitates learning about weak threats. Neuron 93, 164–178 (2017).
Paxinos, G. & Franklin, K. B. J. The Mouse Brain in Stereotaxic Coordinates 2nd edn (Academic Press, 2001).
Tan, K. R. et al. GABA neurons of the VTA drive conditioned place aversion. Neuron 73, 1173–1183 (2012).
Dobi, A., Margolis, E. B., Wang, H. L., Harvey, B. K. & Morales, M. Glutamatergic and nonglutamatergic neurons of the ventral tegmental area establish local synaptic contacts with dopaminergic and nondopaminergic neurons. J. Neurosci. 30, 218–229 (2010).
Xiao, C. et al. Cholinergic mesopontine signals govern locomotion and reward through dissociable midbrain pathways. Neuron 90, 333–347 (2016).
Qi, J. et al. A glutamatergic reward input from the dorsal raphe to ventral tegmental area dopamine neurons. Nat. Commun. 5, 5390 (2014).
Liu, Z. et al. Dorsal raphe neurons signal reward through 5-HT and glutamate. Neuron 81, 1360–1374 (2014).
Poulin, J. F. et al. Mapping projections of molecularly defined dopamine neuron subtypes using intersectional genetic approaches. Nat. Neurosci. 21, 1260–1271 (2018).
Tritsch, N. X., Ding, J. B. & Sabatini, B. L. Dopaminergic neurons inhibit striatal output through non-canonical release of GABA. Nature 490, 262–266 (2012).
Edwards, N. J. et al. Circuit specificity in the inhibitory architecture of the VTA regulates cocaine-induced behavior. Nat. Neurosci. 20, 438–448 (2017).
Liu, Q. S., Pu, L. & Poo, M. M. Repeated cocaine exposure in vivo facilitates LTP induction in midbrain dopamine neurons. Nature 437, 1027–1031 (2005).
Jo, Y. S., Heymann, G. & Zweifel, L. S. Dopamine neurons reflect the uncertainty in fear generalization. Neuron 100, 916–925 (2018).
Burkett, J. P. & Young, L. J. The behavioral, anatomical and pharmacological parallels between social attachment, love and addiction. Psychopharmacology 224, 1–26 (2012).
Barbano, M. F., Wang, H. L., Morales, M. & Wise, R. A. Feeding and reward are differentially induced by activating GABAergic lateral hypothalamic projections to VTA. J. Neurosci. 36, 2975–2985 (2016).
Chuong, A. S. et al. Noninvasive optical inhibition with a red-shifted microbial rhodopsin. Nat. Neurosci. 17, 1123–1129 (2014).
Tian, J. et al. Distributed and mixed information in monosynaptic inputs to dopamine neurons. Neuron 91, 1374–1389 (2016).
Berke, J. D. What does dopamine mean? Nat. Neurosci. 21, 787–793 (2018).
Mameli-Engvall, M. et al. Hierarchical control of dopamine neuron-firing patterns by nicotinic receptors. Neuron 50, 911–921 (2006).
Paladini, C. A. & Tepper, J. M. GABA(A) and GABA(B) antagonists differentially affect the firing pattern of substantia nigra dopaminergic neurons in vivo. Synapse 32, 165–176 (1999).
Faget, L. et al. Opponent control of behavioral reinforcement by inhibitory and excitatory projections from the ventral pallidum. Nat. Commun. 9, 849 (2018).
Parker, J. G. et al. Absence of NMDA receptors in dopamine neurons attenuates dopamine release but not conditioned approach during Pavlovian conditioning. Proc. Natl Acad. Sci. USA 107, 13491–13496 (2010).
Nieh, E. H. et al. Decoding neural circuits that control compulsive sucrose seeking. Cell 160, 528–541 (2015).
Heymann, G. et al. Synergy of distinct dopamine projection populations in behavioral reinforcement. Neuron 105, 909–920 (2020). e905.
Gunaydin, L. A. et al. Natural neural projection dynamics underlying social behavior. Cell 157, 1535–1551 (2014).
Vega-Quiroga, I., Yarur, H. E. & Gysling, K. Lateral septum stimulation disinhibits dopaminergic neurons in the antero-ventral region of the ventral tegmental area: role of GABA-A alpha 1 receptors. Neuropharmacology 128, 76–85 (2018).
Parker, J. G., Beutler, L. R. & Palmiter, R. D. The contribution of NMDA receptor signaling in the corticobasal ganglia reward network to appetitive Pavlovian learning. J. Neurosci. 31, 11362–11369 (2011).
Ting, J. T., Daigle, T. L., Chen, Q. & Feng, G. Acute brain slice methods for adult and aging animals: application of targeted patch clamp analysis and optogenetics. Methods Mol. Biol. 1183, 221–242 (2014).
Acknowledgements
We thank members of the Zweifel lab for scientific discussion on the design and implementation of experiments. We also thank J. Allen for assistance in the production of AAV viral vectors. This work was funded by the US National Institutes of Health (grant nos. P50MH10642, R01-MH104450 and R01-DA044315 to L.S.Z).
Author information
Authors and Affiliations
Contributions
M.E.S., A.S.C., J.M.R. and L.S.Z. designed the experiments, and collected and analyzed the data. M.E.S., A.S.C. and J.M.R. performed the viral injection surgery. M.E.S. and B.C. performed the behavioral analysis. M.E.S. performed the slice electrophysiology. M.E.S., A.S.C., B.C., J.M.R. and L.S.Z. performed the histology and cell counts. L.S.Z. generated CAV2-FLEX-ZsGreen. L.S.Z. and M.E.S purified all viral vectors. R.A. provided the ThFlpO mouse line. M.E.S. and L.S.Z. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Neuroscience thanks Alexxai Kravitz and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Distribution of CAV2-FLEX-zsGreen retrogradely labeled neurons.
Cell counts of retrogradely labeled cells in indicated Cre lines across the rostral-caudal axis. Note that cells from different Cre lines in the same region are plotted on different axes. (Vgat n = 11 mice, Vglut1 n = 4; Vglut2 n = 10; Vglut3 n = 5, 5-HT n = 13, Chat n = 4.) Final panel depicts counts of infected cells in the VTA and neighboring substantia nigra (SN) region (Vgat n = 11 mice, Vglut2 n = 10 mice, One way ANOVA F(3,38)=112.3 p < 0.0001, Tukey’s Multiple Comparison ***p < 0.001). Error bars represent s.e.m.
Extended Data Fig. 2 Retrobead labeling of cholinergic neurons in the PPTg and LDTg.
(a) Representative image of CAV2-FLEX-zsGreen retrogradely labeled cells in the PPTg and LDTg of a Chat Cre mouse. (b) Total cells labeled in the PPTg and LDTg in different Cre driver lines (Vglut2 n = 10, Vgat n = 11, Chat n = 4; PPTg, One-way ANOVA, F(2,28)=7.740, P = 0.0023, *P < 0.05, **P < 0.01; LDTg, One-way ANOVA, F(2,28)=17.41, P < 0.0001, ***P < 0.01, ****P < 0.0001). (c, d) Representative images of Chat Cre:Tomato cells (red) and RetroBeads from the VTA (cyan) in the PPTg and LDTg, and quantification of cell counts and overlap across the rostral-caudal axis (n = 3/group). Error bars represent s.e.m. Scale bars = 50μm.
Extended Data Fig. 3 Retrobead and in situ labeling of VTA inputs.
(a–c) Representative images of indicated regions containing RetroBeads transported from the VTA and RNAscope in situ against Slc32a1 (Vgat) or Slc17a6 (Vglut2) and quantification of the percent of RetroBead labeled cells that colabel with in situ probes for BNST(a), LPO(b), and PAG(c). Arrows identify RetroBead-labeled Vglut-positive neurons, while arrowheads identify RetroBead-labeled Vgat-positive neurons (n = 3 mice, cells counted in 3–5 sections per region per mouse. One-way ANOVA, BNST: F(3,8)=2140, P < 0.0001, LPO: F(3,8)=441.3, P < 0.0001, PAG: F(3,8)=48.31, P < 0.0001, Tukey’s Multiple Comparisons *P < 0.05, **P < 0.01, ***P < 0.001). (d) Representative CAV2-FLEX-ZsGreen labeling in DR of ePet1-Cre mice (scale bar = 100 μm) and cell counts in DR for ePet1, Vglut2, Vglut3, and Vgat Cre mice (ePet n = 13, Vglut2 n = 10, Vglut3 n = 5, Vgat n = 11 mice; One-way ANOVA, F(3,35)=12.10, P = 0.0001, Tukey’s Multiple Comparisons *P < 0.05, **P < 0.01). (e, f) Representative images for DR RetroBeads transported from the VTA and RNAscope in situ against Slc32a1 (Vgat), Slc17a6 (Vglut2), or Slc17a8 (Vglut3), and quantification of the percent of RetroBead labeled cells that colabel with in situ probes. Arrows identify RetroBead-labeled Vglut-positive neurons, while arrowheads identify RetroBead-labeled Vgat-positive neurons (n = 3 mice, cells counted in 3–5 sections per mouse. One-way ANOVA, Vglut2: F(3,8)=161.9, P < 0.0001, Vglut3: F(3,8)=28.94, P = 0.0001, Tukey’s Multiple Comparisons *P < 0.05, **P < 0.01, ***P < 0.001). Error bars represent s.e.m. Scale bars = 50μm.
Extended Data Fig. 4 Density of inputs to the VTA.
(a) Integrated density (arbitrary units) of GFP fluoresence in the VTA following injection of synaptophysinGFP into the indicated region. Red bars: Vgat Cre, blue bars: Vglut 1 or 2 Cre (n = 3 mice/group for all regions except n = 4 mice for LS, NAc core, and BNST). (b) Correlation between average total integrated fluoresence density in the VTA and the average number of cells retrogradely labeled by CAV-FLEX-ZsGreen in each region. Black line = linear regression of all points excluding PFC (n = 3 mice/group for all regions except n = 4 mice for LS, NAc core, and BNST; Pearson r = 0.6570, Spearman two-tailed P = 0.0078). (c) Representative images of synaptophysinGFP injection into the dorsal striatum of a Vgat Cre mouse, and terminals in the SNr, adjacent to the VTA. Scale bars = 500 µm (left) and 200 µm (right). (d) Representative images of synaptophysinGFP injection into the PFC and terminals in the VTA-paranigral region and the adjacent pontine nucleus. Error bars represent s.e.m.
Extended Data Fig. 5 Connectivity of VTA inputs.
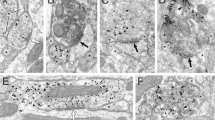
(a) Numbers of connected and not connected Th+ and Th- cells patched in the VTA with ChR2 expressed in the indicated region. Connected cells were those with a visible EPSC detectable across an average of 10 traces. Connected cells in the PFC include those cells that had no visible Li-EPSC until after high frequency stimulation. (b) Percent change in Li-EPSCs amplitude during the first five pulses of a 20 Hz train of light pulses activating PFC or PPTg inputs relative to the first pulse (Two-way RM ANOVA, F(4,60)=3.280, P = 0.0178, Bonferroni multiple comparisons *P < 0.05, ***P < 0.001; PFC n = 8 cells, PPTg n = 9 cells). (c) Percent change in amplitude of Li-EPSCs before and after high frequency stimulation of PFC or PPTg inputs relative to pre-stimulus train amplitude. PFC inputs were stimulated in the presence or absence of AP5 (100 µM), which remained in the bath for the duration of the experiment. (Two-way RM ANOVA, F(14,154)=2.40, P = 0.0046, Bonferroni multiple comparisons vs PPTg *P < 0.05, **P < 0.01; PFC n = 8 cells, PFC + AP5 = 7 cells, PPTg n = 9 cells). (d) Percent of baseline Li-EPSC amplitude following 3×1 s stimulus trains at the indicated frequencies (One-way RM ANOVA, F(3,8)=13.13, P < 0.0001, Tukey’s Multiple Comparisons ***P < 0.001 vs Pre, n = 9 cells). (e) Numbers of connected and not connected Vgat- and Vgat+ cells patched in the VTA with ChR2 expressed in the indicated region. Connected cells were those with a visible IPSC detectable across an average of 10 traces. Error bars represent s.e.m.
Extended Data Fig. 6 Local and distal Vgat knockout.
(a) Example images of RNAscope in situ labeling Cre and Slc32a1 (Vgat) in the VTA following injection of indicated viruses (red=Cre, cyan=Vgat), and quantification of Vgat+ cells/section (n = 3 mice/group, One way ANOVA F(2,6)=77.97 p < 0.0001, Tukey’s multiple comparisons *p < 0.05, ***p < 0.001, scale bar = 100 µm). (b) Locomotor activity (measured as infrared beam breaks) measured in 15 min bins (line) with s.e.m. (shading). (c) Locomotor activity summed over three nights (7 pm to 7 am) and two days (7 am to 7 pm) (Control n = 13 mice, VTA KO n = 11, Distal KO n = 15; Two-way RM ANOVA F(8,144)=6.33, p < 0.0001; Bonferroni multiple comparisons *p < 0.05, ****p < 0.0001). (d) Total head entries during each day of Pavlovian training (n = 23 mice/group control, 21 VTA KO, 27 distal KO; 2-way RM ANOVA F(12,402)=1.9, p = 0.032; Bonferroni multiple comparisons did not achieve significance). (e) Total lever presses during each day of FR1 instrumental conditioning (1 h session/day) (Control n = 21 mice, VTA KO n = 14 mice, Distal KO n = 27 mice; Two-way RM ANOVA F(4,118)=5.35, p = 0.0005; Bonferroni multiple comparisons **p < 0.01). (f) Lever presses on the preferred and non-preferred levers during 3 days of FR1 instrumental conditioning (Control n = 15 mice, VTA KO n = 11 mice, Distal KO n = 15 mice). (g) Percent of time spent freezing during delivery of the CS + or CS- tone during a baseline pretest or following two days of fear conditioning (n = 8 for Control and Distal KO, n = 7 for Local KO; Probe: 2-way RM ANOVA significant effect of CS F(1,20)=19.79 p = 0.0002, Bonferroni multiple comparisons *p < 0.05, **p < 0.01). Error bars represent s.e.m.
Extended Data Fig. 7 Fos induction in TH- cells and Jaws RTPA.
(a) Number of Fos+ TH- cells in each VTA subregion in Vgat-Cre mice expressing YFP or ChR2-YFP in the indicated brain region. (n = 3 mice/group, For IF: One-way ANOVA F(4,10)=9.207, p = 0.002; *p < 0.05, **p < 0.01 vs. YFP.) (b) Real-time place aversion assay comparing percent of time spent in the light paired chamber during the pretest (baseline) period and during the light stimulation period for Vgat-Cre mice expressing YFP or Jaws-GFP in the indicated regions (n = 8 for all groups except n = 10 for LH). Error bars represent s.e.m.
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2 and Supplementary Figs. 1–7.
Rights and permissions
About this article
Cite this article
Soden, M.E., Chung, A.S., Cuevas, B. et al. Anatomic resolution of neurotransmitter-specific projections to the VTA reveals diversity of GABAergic inputs. Nat Neurosci 23, 968–980 (2020). https://doi.org/10.1038/s41593-020-0657-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-020-0657-z
This article is cited by
-
Paternal cocaine-seeking motivation defines offspring’s vulnerability to addiction by down-regulating GABAergic GABRG3 in the ventral tegmental area
Translational Psychiatry (2024)
-
Circuit coordination of opposing neuropeptide and neurotransmitter signals
Nature (2023)
-
Dopamine, behavior, and addiction
Journal of Biomedical Science (2021)
-
Ventral tegmental area GABAergic inhibition of cholinergic interneurons in the ventral nucleus accumbens shell promotes reward reinforcement
Nature Neuroscience (2021)