Abstract
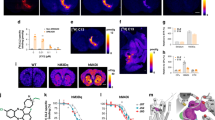
The chemogenetic technology designer receptors exclusively activated by designer drugs (DREADDs) afford remotely reversible control of cellular signaling, neuronal activity and behavior. Although the combination of muscarinic-based DREADDs with clozapine-N-oxide (CNO) has been widely used, sluggish kinetics, metabolic liabilities and potential off-target effects of CNO represent areas for improvement. Here, we provide a new high-affinity and selective agonist deschloroclozapine (DCZ) for muscarinic-based DREADDs. Positron emission tomography revealed that DCZ selectively bound to and occupied DREADDs in both mice and monkeys. Systemic delivery of low doses of DCZ (1 or 3 μg per kg) enhanced neuronal activity via hM3Dq within minutes in mice and monkeys. Intramuscular injections of DCZ (100 μg per kg) reversibly induced spatial working memory deficits in monkeys expressing hM4Di in the prefrontal cortex. DCZ represents a potent, selective, metabolically stable and fast-acting DREADD agonist with utility in both mice and nonhuman primates for a variety of applications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Code availability
The code to generate the results and the figures of this study are available from the corresponding authors upon reasonable request.
References
Armbruster, B. N., Li, X., Pausch, M. H., Herlitze, S. & Roth, B. L. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl Acad. Sci. USA 104, 5163–5168 (2007).
Vardy, E. et al. A new DREADD facilitates the multiplexed chemogenetic interrogation of behavior. Neuron 86, 936–946 (2015).
Roth, B. L. DREADDs for neuroscientists. Neuron 89, 683–694 (2016).
Grayson, D. S. et al. The rhesus monkey connectome predicts disrupted functional networks resulting from pharmacogenetic inactivation of the amygdala. Neuron 91, 453–466 (2016).
Eldridge, M. A. G. et al. Chemogenetic disconnection of monkey orbitofrontal and rhinal cortex reversibly disrupts reward value. Nat. Neurosci. 19, 37–39 (2015).
Nagai, Y. et al. PET imaging-guided chemogenetic silencing reveals a critical role of primate rostromedial caudate in reward evaluation. Nat. Commun. 7, 13605 (2016).
Upright, N. A. et al. Behavioral effect of chemogenetic inhibition is directly related to receptor transduction levels in rhesus monkeys. J. Neurosci. 38, 1418–1422 (2018).
Gomez, J. L. et al. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science 357, 503–507 (2017).
Manvich, D. F. et al. The DREADD agonist clozapine N-oxide (CNO) is reverse-metabolized to clozapine and produces clozapine-like interoceptive stimulus effects in rats and mice. Sci. Rep. 8, 3840 (2018).
Raper, J. et al. Metabolism and distribution of clozapine-N-oxide: implications for nonhuman primate chemogenetics. ACS Chem. Neurosci. 8, 1570–1576 (2017).
Roth, B. L., Sheffler, D. J. & Kroeze, W. K. Magic shotguns versus magic bullets: selectively non-selective drugs for mood disorders and schizophrenia. Nat. Rev. Drug Discov. 3, 353–359 (2004).
Chen, X. et al. The first structure–activity relationship studies for designer receptors exclusively activated by designer drugs. ACS Chem. Neurosci. 6, 476–484 (2015).
Thompson, K. J. et al. DREADD agonist 21 is an effective agonist for muscarinic-based DREADDs in vitro and in vivo. ACS Pharmacol. Transl Sci. 1, 61–72 (2018).
Phillips, S. T. et al. Binding of 5H-dibenzo[b,e][1,4]diazepine and chiral 5H-dibenzo[a,d]cycloheptene analogs of clozapine to dopamine and serotonin receptors. J. Med. Chem. 37, 2686–2696 (1994).
Ji, B. et al. Multimodal imaging for DREADD-expressing neurons in living brain and their application to implantation of iPSC-derived neural progenitors. J. Neurosci. 36, 11544–11558 (2016).
Farrell, M. S. & Roth, B. L. Pharmacosynthetics: reimagining the pharmacogenetic approach. Brain Res. 1511, 6–20 (2013).
Kalvass, J. C., Maurer, T. S. & Pollack, G. M. Use of plasma and brain unbound fractions to assess the extent of brain distribution of 34 drugs: comparison of unbound concentration ratios to in vivo P-glycoprotein efflux ratios. Drug Metab. Dispos. 35, 660–666 (2007).
Maurer, T. S., DeBartolo, D. B., Tess, D. A. & Scott, D. O. Relationship between exposure and nonspecific binding of thirty-three central nervous system drugs in mice. Drug Metab. Dispos. 33, 175–181 (2005).
Martinez, M. N. Factors influencing the use and interpretation of animal models in the development of parenteral drug delivery systems. AAPS J. 13, 632–649 (2011).
Olsen, R.H.J. et al. TRUPATH, an open-source biosensor platform for interrogating the GPCR transducerome. Nat. Chem. Biol. https://doi.org/10.1038/s41589-020-0535-8 (2020).
Kroeze, W. K. et al. PRESTO-Tango as an open-source resource for interrogation of the druggable human GPCRome. Nat. Struct. Mol. Biol. 22, 362–369 (2015).
Alexander, G. M. et al. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron 63, 27–39 (2009).
Buzsáki, G., Anastassiou, C. A. & Koch, C. The origin of extracellular fields and currents—EEG, ECoG, LFP and spikes. Nat. Rev. Neurosci. 13, 407–420 (2012).
Michaelides, M. et al. Whole-brain circuit dissection in free-moving animals reveals cell-specific mesocorticolimbic networks. J. Clin. Invest. 123, 5342–5350 (2013).
Phelps, M. E. et al. Tomographic measurement of local cerebral glucose metabolic rate in humans with (F-18)2-fluoro-2-deoxy-d-glucose: validation of method. Ann. Neurol. 6, 371–388 (1979).
Poremba, A. et al. Species-specific calls evoke asymmetric activity in the monkey’s temporal poles. Nature 427, 448–451 (2004).
Tsutsui, K.-I., Oyama, K., Nakamura, S. & Iijima, T. Comparative overview of visuospatial working memory in monkeys and rats. Front. Syst. Neurosci. 10, 99 (2016).
Allen, D. C. et al. A comparative study of the pharmacokinetics of clozapine N-oxide and clozapine N-oxide hydrochloride salt in rhesus macaques. J. Pharmacol. Exp. Ther. 368, 199–207 (2018).
Kätzel, D., Nicholson, E., Schorge, S., Walker, M. C. & Kullmann, D. M. Chemical–genetic attenuation of focal neocortical seizures. Nat. Commun. 5, 3847 (2014).
Magnus, C. J. et al. Ultrapotent chemogenetics for research and potential clinical applications. Science 364, eaav5282 (2019).
Szablowski, J. O., Lee-Gosselin, A., Lue, B., Malounda, D. & Shapiro, M. G. Acoustically targeted chemogenetics for the non-invasive control of neural circuits. Nat. Biomed. Eng. 2, 475–484 (2018).
Stachniak, T. J., Ghosh, A. & Sternson, S. M. Chemogenetic synaptic silencing of neural circuits localizes a hypothalamus→midbrain pathway for feeding behavior. Neuron 82, 797–808 (2014).
Tomita, Y. et al. Long-term in vivo investigation of mouse cerebral microcirculation by fluorescence confocal microscopy in the area of focal ischemia. J. Cereb. Blood Flow Metab. 25, 858–867 (2005).
Tajima, Y. et al. Changes in cortical microvasculature during misery perfusion measured by two-photon laser scanning microscopy. J. Cereb. Blood Flow Metab. 34, 1363–1372 (2014).
Fridén, M. et al. Measurement of unbound drug exposure in brain: modeling of pH partitioning explains diverging results between the brain slice and brain homogenate methods. Drug Metab. Dispos. 39, 353–362 (2011).
Bender, D., Holschbach, M. & Stöcklin, G. Synthesis of n.c.a. carbon-11 labelled clozapine and its major metabolite clozapine-N-oxide and comparison of their biodistribution in mice. Nucl. Med. Biol. 21, 921–925 (1994).
Kiebel, S. J., Ashburner, J., Poline, J.-B. & Friston, K. J. MRI and PET coregistration—a cross validation of statistical parametric mapping and automated image registration. NeuroImage 5, 271–279 (1997).
Takuwa, H. et al. Reproducibility and variance of a stimulation-induced hemodynamic response in barrel cortex of awake behaving mice. Brain Res. 1369, 103–111 (2011).
Croxson, P. L., Kyriazis, D. A. & Baxter, M. G. Cholinergic modulation of a specific memory function of prefrontal cortex. Nat. Neurosci. 14, 1510–1512 (2011).
Goldman, P. S., Rosvold, E. H. & Mishkin, M. Evidence for behavioral impairment following prefrontal lobectomy in the infant monkey. J. Comp. Physiol. Psychol. 70, 454–463 (1970).
Minamimoto, T., La Camera, G. & Richmond, B. J. Measuring and modeling the interaction among reward size, delay to reward, and satiation level on motivation in monkeys. J. Neurophysiol. 101, 437–447 (2008).
Ichise, M. et al. Noninvasive quantification of dopamine D2 receptors with iodine-123-IBF SPECT. J. Nucl. Med. 37, 513–520 (1996).
McLaren, D. G. et al. A population-average MRI-based atlas collection of the rhesus macaque. NeuroImage 45, 52–59 (2009).
Acknowledgements
We thank R. Suma, J. Kamei, R. Yamaguchi, Y. Matsuda, Y. Sugii, A. Maruyama, T. Okauchi, T. Kokufuta, Y. Iwasawa, T. Watanabe, A. Tanizawa, S. Shibata, N. Nitta, Y. Ozawa, M. Fujiwara, M. Nakano, T. Minamihisamatsu, S. Uchida and S. Sasaki for their technical assistance. We also thank S. Hiura for 3D printing of the grids. This study was supported by the following grants and organizations: MEXT/JSPS KAKENHI grant numbers JP15H05917, JP15K12772 and JP18H04037 (to T.M.), JP16H02454 (to M. Takada), JP19K08138 (to Y.N.), and JP18H05018, JP19K07811 and JP20H04596 (to N.M.); AMED grant numbers JP20dm0107146 (to T.M.), JP19dm0207003 (to M. Takada), JP20dm0107094 and JP18dm0207007 (to T.S.), JP20dm0307021 (to K.-i.I.), JP20dm0307007 (to T.H.), and JP20dm0207072 (to M.H.); JST PRESTO grant number JPMJPR1683 (to K.-i.I.); QST President’s Strategic Grant (Creative Research) (to N.M.); the Cooperative Research Program at PRI, Kyoto University; the National Bio-Resource Project ‘Japanese Monkeys’ of MEXT, Japan; and U24DK116195, the NIMH Psychoactive Drug Screening Program and the Michael Hooker Distinguished Professorship to B.L.R.
Author information
Authors and Affiliations
Contributions
Conceptualization: T.M. Formal analysis: Y.N., N.M., K.O., K.M. and T.M. Investigation: Y.N., N.M., H.T., Y.H., K.O., B.J., M. Takahashi, X.-P.H., S.T.S., J.F.D., T.U., A.F., J.G.E., K.K., C.S., M.O. and M.S. Resources: B.J., Y.X., J.L., K.-i.I., Y.T., J.N., M. Takada and J.J. Writing (original draft): Y.N., N.M. and T.M. Visualization: Y.N., N.M., H.T., Y.H., K.O., B.J., K.M. and T.M. Supervision: M.-R.Z., T.S., M. Takada, M.H., J.J., B.L.R. and T.M. Project administration: B.L.R. and T.M. Funding acquisition: Y.N., N.M., T.H., K.I., M. Takada, T.S., M.H., J.J., B.L.R., and T.M. Writing (review and editing): all authors.
Corresponding authors
Ethics declarations
Competing interests
Y.N., N.M., B.J., T.S., M.H. and T.M. are named as inventors on a patent application (PCT/JP2019/024834; status: patent pending) claiming subject matter related to the results described in this paper. The remaining authors declare no competing interests.
Additional information
Peer review information Nature Neuroscience thanks Mikhail Shapiro and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–6 and Supplementary Tables 1 and 2.
Rights and permissions
About this article
Cite this article
Nagai, Y., Miyakawa, N., Takuwa, H. et al. Deschloroclozapine, a potent and selective chemogenetic actuator enables rapid neuronal and behavioral modulations in mice and monkeys. Nat Neurosci 23, 1157–1167 (2020). https://doi.org/10.1038/s41593-020-0661-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-020-0661-3
This article is cited by
-
Persistent enhancement of basolateral amygdala-dorsomedial striatum synapses causes compulsive-like behaviors in mice
Nature Communications (2024)
-
Specification of neural circuit architecture shaped by context-dependent patterned LAR-RPTP microexons
Nature Communications (2024)
-
Synthesis and preclinical evaluation of [11C]uPSEM792 for PSAM4-GlyR based chemogenetics
Scientific Reports (2024)
-
Psychedelics: preclinical insights provide directions for future research
Neuropsychopharmacology (2024)
-
Activation of prefrontal parvalbumin interneurons ameliorates working memory deficit even under clinically comparable antipsychotic treatment in a mouse model of schizophrenia
Neuropsychopharmacology (2024)