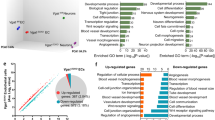
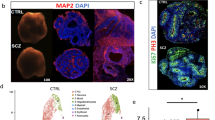
Abstract
While the neuronal underpinnings of autism spectrum disorder (ASD) are being unraveled, vascular contributions to ASD remain elusive. Here, we investigated postnatal cerebrovascular development in the 16p11.2df/+ mouse model of 16p11.2 deletion ASD syndrome. We discover that 16p11.2 hemizygosity leads to male-specific, endothelium-dependent structural and functional neurovascular abnormalities. In 16p11.2df/+ mice, endothelial dysfunction results in impaired cerebral angiogenesis at postnatal day 14, and in altered neurovascular coupling and cerebrovascular reactivity at postnatal day 50. Moreover, we show that there is defective angiogenesis in primary 16p11.2df/+ mouse brain endothelial cells and in induced-pluripotent-stem-cell-derived endothelial cells from human carriers of the 16p11.2 deletion. Finally, we find that mice with an endothelium-specific 16p11.2 deletion (16p11.2ΔEC) partially recapitulate some of the behavioral changes seen in 16p11.2 syndrome, specifically hyperactivity and impaired motor learning. By showing that developmental 16p11.2 haploinsufficiency from endothelial cells results in neurovascular and behavioral changes in adults, our results point to a potential role for endothelial impairment in ASD.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Source data for the bulk RNA-seq experiments are available (GSE147790), and information on iPSC lines can be found in Supplementary Table 1. More details on control lines are available from the Stanford Lab (wstanford@ohri.ca). ANOVA tables are given as statistics source data files. All other data and protocols are available from the corresponding author upon reasonable request. Source data are provided with this paper.
Code availability
The custom scripts for blood vessel and neuronal quantifications, written in Python, are available on GitHub (https://github.com/chcomin/NatNeurosci2020) and from the Comin Lab (chcomin@gmail.com).
References
Walsh, J. J. et al. 5-HT release in nucleus accumbens rescues social deficits in mouse autism model. Nature 560, 589–594 (2018).
Ebert, D. H. & Greenberg, M. E. Activity-dependent neuronal signalling and autism spectrum disorder. Nature 493, 327–337 (2013).
Attwell, D. & Laughlin, S. B. An energy budget for signaling in the grey matter of the brain. J. Cereb. Blood Flow. Metab. 21, 1133–1145 (2001).
Lacoste, B. et al. Sensory-related neural activity regulates the structure of vascular networks in the cerebral cortex. Neuron 83, 1117–1130 (2014).
Segarra, M. et al. Endothelial Dab1 signaling orchestrates neuro–glia–vessel communication in the central nervous system. Science 361, eaao2861 (2018).
Andreone, B. J., Lacoste, B. & Gu, C. Neuronal and vascular interactions. Annu. Rev. Neurosci. 38, 25–46 (2015).
Kisler, K., Nelson, A. R., Montagne, A. & Zlokovic, B. V. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 18, 419–434 (2017).
Sweeney, M. D., Sagare, A. P. & Zlokovic, B. V. Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 14, 133–150 (2018).
Azmitia, E. C., Saccomano, Z. T., Alzoobaee, M. F., Boldrini, M. & Whitaker-Azmitia, P. M. Persistent angiogenesis in the autism brain: an immunocytochemical study of postmortem cortex, brainstem and cerebellum. J. Autism Dev. Disord. 46, 1307–1318 (2016).
Jann, K. et al. Altered resting perfusion and functional connectivity of default mode network in youth with autism spectrum disorder. Brain Behav. 5, e00358 (2015).
Cook, E. H. Jr & Scherer, S. W. Copy-number variations associated with neuropsychiatric conditions. Nature 455, 919–923 (2008).
Steinberg, S. et al. Common variant at 16p11.2 conferring risk of psychosis. Mol. Psychiatry 19, 108–114 (2014).
Zheng, X. et al. The association between rare large duplication of 16p11.2 and schizophrenia in the Singaporean Chinese population. Schizophr. Res. 146, 368–369 (2013).
Weiss, L. A. et al. Association between microdeletion and microduplication at 16p11.2 and autism. N. Engl. J. Med. 358, 667–675 (2008).
Simons VIP Connect Study Team. 16p11.2 Deletion Syndrome Guidebook https://diazatienza.es/wp-content/uploads/2017/12/16p_GUIDEBOOK_FINAL_VERSION.pdf (Simons VIP Connect, 2015).
Hippolyte, L. et al. The number of genomic copies at the 16p11.2 locus modulates language, verbal memory, and inhibition. Biol. Psychiatry 80, 129–139 (2016).
Blackmon, K. et al. Focal cortical anomalies and language impairment in 16p11.2 deletion and duplication syndrome. Cereb. Cortex 28, 2422–2430 (2018).
Owen, J. P. et al. Aberrant white matter microstructure in children with 16p11.2 deletions. J. Neurosci. 34, 6214–6223 (2014).
Horev, G. et al. Dosage-dependent phenotypes in models of 16p11.2 lesions found in autism. Proc. Natl Acad. Sci. USA 108, 17076–17081 (2011).
Portmann, T. et al. Behavioral abnormalities and circuit defects in the basal ganglia of a mouse model of 16p11.2 deletion syndrome. Cell Rep. 7, 1077–1092 (2014).
Yang, M. et al. 16p11.2 Deletion syndrome mice display sensory and ultrasonic vocalization deficits during social interactions. Autism Res. 8, 507–521 (2015).
Tian, D. et al. Contribution of mGluR5 to pathophysiology in a mouse model of human chromosome 16p11.2 microdeletion. Nat. Neurosci. 18, 182–184 (2015).
Reynell, C. & Harris, J. J. The BOLD signal and neurovascular coupling in autism. Dev. Cogn. Neurosci. 6, 72–79 (2013).
Needles, A. et al. Nonlinear contrast imaging with an array-based micro-ultrasound system. Ultrasound Med. Biol. 36, 2097–2106 (2010).
Fischer, K. et al. Testing the efficacy of contrast-enhanced ultrasound in detecting transplant rejection using a murine model of heart transplantation. Am. J. Transplant. 17, 1791–1801 (2017).
Kozberg, M. G., Ma, Y., Shaik, M. A., Kim, S. H. & Hillman, E. M. Rapid postnatal expansion of neural networks occurs in an environment of altered neurovascular and neurometabolic coupling. J. Neurosci. 36, 6704–6717 (2016).
Chen, B. R., Kozberg, M. G., Bouchard, M. B., Shaik, M. A. & Hillman, E. M. A critical role for the vascular endothelium in functional neurovascular coupling in the brain. J. Am. Heart Assoc. 3, e000787 (2014).
Hillman, E. M. Coupling mechanism and significance of the BOLD signal: a status report. Annu. Rev. Neurosci. 37, 161–181 (2014).
Mannell, H. K. et al. ARNO regulates VEGF-dependent tissue responses by stabilizing endothelial VEGFR-2 surface expression. Cardiovasc. Res. 93, 111–119 (2012).
Harb, R., Whiteus, C., Freitas, C. & Grutzendler, J. In vivo imaging of cerebral microvascular plasticity from birth to death. J. Cereb. Blood Flow. Metab. 33, 146–156 (2013).
Mitola, S. et al. Gremlin is a novel agonist of the major proangiogenic receptor VEGFR2. Blood 116, 3677–3680 (2010).
Dutton, L. R., O’Neill, C. L., Medina, R. J. & Brazil, D. P. No evidence of Gremlin1-mediated activation of VEGFR2 signaling in endothelial cells. J. Biol. Chem. 294, 18041–18045 (2019).
Ma, B., Kang, Q., Qin, L., Cui, L. & Pei, C. TGF-β2 induces transdifferentiation and fibrosis in human lens epithelial cells via regulating gremlin and CTGF. Biochem. Biophys. Res. Commun. 447, 689–695 (2014).
Zode, G. S., Clark, A. F. & Wordinger, R. J. Bone morphogenetic protein 4 inhibits TGF-β2 stimulation of extracellular matrix proteins in optic nerve head cells: role of gremlin in ECM modulation. Glia 57, 755–766 (2009).
Angelakos, C. C. et al. Hyperactivity and male-specific sleep deficits in the 16p11.2 deletion mouse model of autism. Autism Res. 10, 572–584 (2017).
Yadav, S. et al. TAOK2 kinase mediates PSD95 stability and dendritic spine maturation through septin7 phosphorylation. Neuron 93, 379–393 (2017).
Ip, J. P. K. et al. Major vault protein, a candidate gene in 16p11.2 microdeletion syndrome, is required for the homeostatic regulation of visual cortical plasticity. J. Neurosci. 38, 3890–3900 (2018).
Shin, M. et al. Vegfa signals through ERK to promote angiogenesis, but not artery differentiation. Development 143, 3796–3805 (2016).
Anderson, A. W. et al. Neonatal auditory activation detected by functional magnetic resonance imaging. Magn. Reson. Imaging 19, 1–5 (2001).
Wen, T. H., Lovelace, J. W., Ethell, I. M., Binder, D. K. & Razak, K. A. Developmental changes in EEG phenotypes in a mouse model of fragile X syndrome. Neuroscience 398, 126–143 (2019).
Berman, J. I. et al. Relationship between M100 auditory evoked response and auditory radiation microstructure in 16p11.2 deletion and duplication carriers. Am. J. Neuroradiol. 37, 1178–1184 (2016).
Miyazaki-Akita, A. et al. 17β-estradiol antagonizes the down-regulation of endothelial nitric-oxide synthase and GTP cyclohydrolase I by high glucose: relevance to postmenopausal diabetic cardiovascular disease. J. Pharmacol. Exp. Ther. 320, 591–598 (2007).
Grissom, N. M. et al. Male-specific deficits in natural reward learning in a mouse model of neurodevelopmental disorders. Mol. Psychiatry 23, 544–555 (2018).
Gur, R. C. et al. Sex and handedness differences in cerebral blood flow during rest and cognitive activity. Science 217, 659–661 (1982).
Ospina, J. A., Duckles, S. P. & Krause, D. N. 17β-estradiol decreases vascular tone in cerebral arteries by shifting COX-dependent vasoconstriction to vasodilation. Am. J. Physiol. Heart Circ. Physiol. 285, H241–H250 (2003).
Robinson, E. B., Lichtenstein, P., Anckarsater, H., Happe, F. & Ronald, A. Examining and interpreting the female protective effect against autistic behavior. Proc. Natl Acad. Sci. USA 110, 5258–5262 (2013).
Goldman, S. A. & Chen, Z. Perivascular instruction of cell genesis and fate in the adult brain. Nat. Neurosci. 14, 1382–1389 (2011).
Tata, M. & Ruhrberg, C. Cross-talk between blood vessels and neural progenitors in the developing brain. Neuronal Signal. 2, NS20170139 (2018).
Flygare Wallen, E., Ljunggren, G., Carlsson, A. C., Pettersson, D. & Wandell, P. High prevalence of diabetes mellitus, hypertension and obesity among persons with a recorded diagnosis of intellectual disability or autism spectrum disorder. J. Intellect. Disabil. Res. 62, 269–280 (2018).
Sigmon, E. R., Kelleman, M., Susi, A., Nylund, C. M. & Oster, M. E. Congenital heart disease and autism: a case–control study. Pediatrics 144, e20184114 (2019).
Alva, J. A. et al. VE-cadherin-Cre-recombinase transgenic mouse: a tool for lineage analysis and gene deletion in endothelial cells. Dev. Dyn. 235, 759–767 (2006).
Tsai, H. H. et al. Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science 337, 358–362 (2012).
Tunster, S. J. Genetic sex determination of mice by simplex PCR. Biol. Sex. Differ. 8, 31 (2017).
Munoz, N. M. et al. Comparison of dynamic contrast-enhanced magnetic resonance imaging and contrast-enhanced ultrasound for evaluation of the effects of sorafenib in a rat model of hepatocellular carcinoma. Magn. Reson. Imaging 57, 156–164 (2019).
Lacoste, B., Tong, X. K., Lahjouji, K., Couture, R. & Hamel, E. Cognitive and cerebrovascular improvements following kinin B1 receptor blockade in Alzheimer’s disease mice. J. Neuroinflammation 10, 57 (2013).
Lovelace, J. W., Ethell, I. M., Binder, D. K. & Razak, K. A. Translation-relevant EEG phenotypes in a mouse model of fragile X syndrome. Neurobiol. Dis. 115, 39–48 (2018).
Tong, X. K., Nicolakakis, N., Kocharyan, A. & Hamel, E. Vascular remodeling versus amyloid β-induced oxidative stress in the cerebrovascular dysfunctions associated with Alzheimer’s disease. J. Neurosci. 25, 11165–11174 (2005).
Thibodeau, J. F. et al. Vascular smooth muscle-specific EP4 receptor deletion in mice exacerbates angiotensin II-induced renal injury. Antioxid. Redox Signal. 25, 642–656 (2016).
Lindeberg, T. Feature detection with automatic scale selection. Int. J. Comput. Vis. 30, 79–116 (1998).
Travencolo, B. A. et al. A new method for quantifying three-dimensional interactions between biological structures. J. Anat. 210, 221–231 (2007).
Woodworth, M. B. et al. Ctip1 regulates the balance between specification of distinct projection neuron subtypes in deep cortical layers. Cell Rep. 15, 999–1012 (2016).
Pinto, L. et al. AP2γ regulates basal progenitor fate in a region- and layer-specific manner in the developing cortex. Nat. Neurosci. 12, 1229–1237 (2009).
Tremblay, M. E., Riad, M. & Majewska, A. Preparation of mouse brain tissue for immunoelectron microscopy. J. Vis. Exp. https://doi.org/10.3791/2021 (2010).
Bisht, K., El Hajj, H., Savage, J. C., Sanchez, M. G. & Tremblay, M. E. Correlative light and electron microscopy to study microglial interactions with beta-amyloid plaques. J. Vis. Exp. https://doi.org/10.3791/54060 (2016).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Zerbino, D. R. et al. Ensembl 2018. Nucleic Acids Res. 46, D754–D761 (2018).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Tchagang, A. B. et al. GOAL: a software tool for assessing biological significance of genes groups. BMC Bioinf. 11, 229 (2010).
Tatsumi, R. et al. Simple and highly efficient method for production of endothelial cells from human embryonic stem cells. Cell Transplant. 20, 1423–1430 (2011).
Cao, V. Y. et al. Motor learning consolidates arc-expressing neuronal ensembles in secondary motor cortex. Neuron 86, 1385–1392 (2015).
Behringer, R., Gertsenstein, M., Nagy, K. V. & Nagy, A. Selecting female mice in estrus and checking plugs. Cold Spring Harb. Protoc. https://doi.org/10.1101/pdb.prot092387 (2016).
Angoa-Perez, M., Kane, M. J., Briggs, D. I., Francescutti, D. M. & Kuhn, D. M. Marble burying and nestlet shredding as tests of repetitive, compulsive-like behaviors in mice. J. Vis. Exp. https://doi.org/10.3791/50978 (2013).
Acknowledgements
We thank J.-C. Béïque, C.D. Harvey, P. Kaeser and C. Gu for their valuable comments on the manuscript; E. Hamel for generously sharing pressure myography equipment from her laboratory; D. Lagace, K. Ure and their assistant M. Barclay for training and guidance on behavioral assays; T. Portmann for advice on mouse genetics; C. Boisvert and K. Slodki for technical assistance on mouse husbandry and genotyping; A. Gagné and N. Vernoux for technical assistance on TEM; F. Xiao and M. Munkonda for training J. Ouellette on cell cycle analysis and tail-cuff plethysmography; L. Zhu for technical assistance; D.B. Stanimirovic for facilitating the collaboration with the National Research Council of Canada; A. Heinmiller for sharing equipment from the Fujifilm VisualSonics facility and for guidance on acoustic contrast imaging; S. Thompson for guidance on the marble-burying test; and C. Doré for helping organize experiments using control iPSC lines. For this work, B.L. was supported by start-up funds from the Ottawa Hospital Research Institute, by research grants from the Canadian Institutes of Health Research (CIHR) (grant no. 388805), the Scottish Rite Charitable Foundation of Canada (grant no. 17112), and the J. P. Bickell Foundation. C.H.C. thanks FAPESP (grant no. 15/18942-8). L.d.F.C. thanks CNPq (grant no. 307333/2013-2), FAPESP (grant no. 11/50761-2 and no. 2015/22308-2) and NAP-PRP-USP.
Author information
Authors and Affiliations
Contributions
J.O., X.T., B.L., M.H., M.L.-A., S.L., M.Y., J.-F.T., C.J.M., P.V.D., M.F.-A., M.C., Y.D.B. and C.J.B. performed experiments. J.O., X.T., M.H., C.H.C., L.d.F.C., M.L.-A., C.J.M., J.-F.T., P.V.D., M.F.-A. and C.J.B. analyzed the data/images in a blinded manner. Q.Y.L., S.L., Y.P., Z.L., Y.D.B. and B.L. generated and/or analyzed transcriptomic data. S.B. provided expertise for the ECoG data analysis. W.L.S. provided healthy donor iPSC lines and expertise in stem cell research. D.J.S. (supervisor of M.H.) provided expertise in endothelial differentiation of iPSCs. B.L. conceived and led the project, designed experiments and wrote the manuscript from a draft produced by J.O., with input from X.T., M.F.-A., M.L.-A., M.-È.T., D.B., C.R.K., S.B., Y.D.B., D.J.S. and W.L.S.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Neuroscience thanks Anusha Mishra and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Neurovascular parameters in 16p11.2df/+ and WT mice at P14 and P50.
a, CBF assessment by LDF in 16p11.2df/+ and WT females at P14 and P50. Only falling slope appeared affected by genotype in females at P14. b, Additional representative images and a diagram for contrast imaging method, showing the region of interest (ROI, dotted lines) before (pre.) and after (post.) i.v. injection of microbubbles. The graph on the right shows identical ROI size in all animals. c, Additional CBF parameters in 16p11.2df/+ and WT males versus females. d, LDF traces (mean ± s.e.m.) obtained before, during, and after whisker stimulation in all mice (regrouped by genotype). e, Mean systolic blood pressure and heart rate measured over 5 days at P50 using tail cuffs. WT, Wild-Type. Data are whisker boxes (min to max, center line indicating median) in a and c, or mean with individual values in b and e. Traces in a and d are mean ± s.e.m. (n = 4-8 animals per group). *P < 0.05 (two tailed Mann-Whitney test). ♂: males; ♀: females.
Extended Data Fig. 2 Cerebrovascular and electrophysiological parameters in male and female 16p11.2df/+ and WT mice at P14 and P50.
a, LDF recording (Tissue perfusion units, mean ± s.e.m.) of resting state CBF over the primary somatosensory cortex from anesthetized mice, averaged over 40 sec. b, Quantification and comparison of resting state CBF using LDF in all groups of mice. c,d, ECoG recordings in the primary somatosensory cortex from P14 male (c), and P50 female (d) 16p11.2df/+ and WT mice. In c and d: Left, Representative power spectral traces of low-frequency bands (n = 4-5 animals per group; 6 stimulations per animal). Right, Average absolute power in Delta (1-4 Hz), Theta (4-8 Hz), Alpha (8-13 Hz), Beta (13-30 Hz), Low Gamma (35-55 Hz) and High Gamma (65-100 Hz) frequency bands at resting-state (upper panel) and during stimulation (lower panel). Data (right) are mean with individual values (n = 4-5 animals per group; 6 stimulations per animal). Data are mean ± s.e.m. in a, whisker boxes (min to max, center line indicating median) in b, or mean with individual values in c,d (right) (n = 4-6 animals per group). **P < 0.01, ***P < 0.001 (2-way ANOVA and Tukey’s post-hoc test in b).
Extended Data Fig. 3 Ex vivo vascular reactivity (VR) of middle cerebral and mesenteric arteries from 16p11.2df/+ and WT mice at P50.
a, Schematic representation of cellular and molecular VR mechanisms. b, Upper panels, Wire myography of mesenteric arteries ex vivo confirming 16p11.2 deletion-induced endothelial dysfunction. Females and males display a similar endothelial-dependent deficit, but normal VSMC response. Lower panels, pD2 values obtained from the dose-response curves from male and female mice. c, pD2 values obtained from dose-response curves of male and female middle cerebral arteries (see Fig. 2). ACh, acetylcholine; L-NNA, NG-Nitro-L-arginine; PE, phenylephrine; SNP, sodium nitroprusside; VSMC, vascular smooth muscle cell; WT, Wild-Type. Data are mean ± s.e.m. in b (upper panel), or whisker boxes (min to max, center line indicating median) in b (lower panel) and c (n = 5-7 animals per sex group). *P < 0.05 (2-way repeated measure ANOVA and Tukey’s post-hoc test in b).
Extended Data Fig. 4 Postnatal neurovascular maturation in the cerebral cortex of 16p11.2df/+ and WT mice.
a–c, Postnatal developmental profile of cerebral cortex endothelial networks in 16p11.2df/+ and WT males (cortical layers where most significant differences were found). d-i, Postnatal developmental profile of cerebral cortex endothelial networks in 16p11.2df/+ and WT females. j, Sample image of the computational approach used to delineate ROIs in the cortex to quantify neuronal density (see methods for details). k, Quantification of neuronal density in the parietal (that is, somatosensory) cortex of female mice following immunostaining for neuronal markers NeuN and TBR1. l, Vascular endothelial growth factor-A (VEGF-A) levels measured by E.L.I.S.A. in protein extracts from cerebral cortex micro-dissected at P14 or P50 in male and female mice. WT, Wild-Type. Data are mean ± s.e.m. in a-i and k, or mean with individual values in l (n = 3-6 animals per group). *P < 0.05 (two tailed Mann-Whitney test). #P < 0.05, ###P < 0.001 (2-way ANOVA and Sidak’s post-hoc test).
Extended Data Fig. 5 Morphology of the neurovascular unit in male 16p11.2df/+ and WT mice at P14 and P50.
Immunohistochemical analysis of vascular smooth muscle cells, VSMCs (a, SMA), pericytes (b, PDGFR-β), astrocytes (c, Aldh1l1-eGFP) and microglia (d, Iba1) in the cerebral cortex. a, Endothelial coverage by VSMCs measured in the anterior, parietal and occipital cortex. b, Pericyte density and endothelial coverage measured in the anterior, parietal and occipital cortex. Endothelial marker CD31 was used in a and b to stain vessels. c, Astrocyte density and surface coverage measured in the anterior, parietal and occipital cortex of mice expressing eGFP under the control of the pan-astrocytic Aldh1l1 promotor. d, Microglia density and surface coverage measured in the anterior, parietal and occipital cortex. e, Top, Transmission electron micrograph showing astrocytic endfeet (red-pseudocolored) surrounding a brain capillary. Bottom, Quantification of average endfoot size (left) and endothelial coverage ratio by endfeet (right). f, Transmission electron micrographs showing pericytes (pink-pseudocolored) within the basement membrane around brain endothelial cells (green-pseudocolored). Images are representative of experiments repeated in 4 male mice per group, with similar results. Normal astrocyte coverage and pericyte attachment were observed in 16p11.2df/+ mice. A, astrocytes; L, lumen; P, pericyte; WT, Wild-Type. All data are mean with individual values (n = 3-7 animals per group). *P < 0.05 (two tailed Mann-Whitney test in c).
Extended Data Fig. 6 Additional information on neurovascular features in conditional 16p11.2ΔEC mutants and Cdh5-Cretg/+ controls at P50.
a, Quantification of neuronal density in the parietal (that is, somatosensory) cortex of P50 males and females following immunostaining for neuronal markers NeuN and TBR1. b, Quantification of cortical thickness and layering from micrographs of DAPI-stained brain sections from males and females. No difference was evidenced between 16p11.2ΔEC and control mice. c, Normal mean systolic blood pressure and heart rate in 16p11.2ΔEC as measured by tail cuffs. d, ECoG recordings in the primary somatosensory cortex from P50 female 16p11.2ΔEC and control mice. Top panels, representative power spectral traces of low-frequency bands. Bottom graphs, average absolute power in Delta (1-4 Hz), Theta (4-8 Hz), Alpha (8-13 Hz), Beta (13-30 Hz), Low Gamma (35-55 Hz) and High Gamma (65-100 Hz) frequency bands at resting-state (upper graphs) and during stimulation (bottom graphs). Data are mean ± s.e.m. in a, whisker boxes (min to max, center line indicating median) in b, or mean with individual values in c and d (n = 4-8 animals per group). #P < 0.05 (2-way ANOVA and Sidak’s post-hoc test in a). *P < 0.05 (Mann-Whitney test in c).
Extended Data Fig. 7 Characterization of primary mouse cerebral cortex ECs (cECs) from male WT and 16p11.2df/+ mice.
a, Representative images and quantifications of immunocytochemical staining for cEC-specific markers GLUT-1, eNOS and VE-Cadherin, showing no difference between WT and mutant cECs isolated at P14 (blue: DAPI). b, Assessment of apoptosis in P14 cEC cultures. The Caspase-3/7 green assay revealed normal apoptotic rates in 16p11.2df/+ cECs. c, qPCR validation on cEC RNA using mouse VEGFR-2, CD31 and eNOS as markers (a no reverse transcriptase control was used as negative control). d, Assessment of endothelial gene enrichment using RNAseq data normalized to a publicly-available database from Dr. Ben A. Barres lab, Stanford University, USA (Zhang et al. 2014, PMID 25186741; http://www.brainrnaseq.org/). e, Assessment of neuronal contamination using RNAseq data (as in d). A very low level of contamination was achieved. Examples given are from cortical endothelial cells (cECs) isolated from male mice at P14. f, Confirmation of cEC 16p11.2 haploinsufficiency by RNAseq. Mapping of fold change (FC) to 7qF3 locus genes confirms a ~50% decrease in gene expression levels at both P14 and P50. Data are mean ± s.e.m in a (VE-Cadh.) and c,d,e, whisker boxes (min to max, center line indicating median) in a (eNOS, GLUT1), or mean with individual values in b. CTL, control; WT, Wild-Type. For RNAseq, n = 3-4 biological replicates per group (2 mice per replicate).
Extended Data Fig. 8 In vitro network formation assay using primary cECs from P14 and P50 male mice.
a, In vitro network formation assay using primary cECs from P50 brains to assess vascular network formation and remodeling over 48 hrs in a growth factor-reduced Matrigel® (EGF < 0.5 ng/mL; PDGF < 5 pg/mL; IGF-1 5 ng/mL; TGF-β 1.7 ng/mL). No significant difference was quantified between 16p11.2df/+ and WT cECs. b, Assessment of cell proliferation using cell cycle analysis with cECs from P50 brains. The proportion of cells in G2/S (proliferation) or G1 (growth) phases was identical between 16p11.2df/+ and WT cECs. c, Cultured P14 cECs were seeded in a growth-factor supplemented Matrigel® (EGF: 0.7 ng/mL; PDGF 12 pg/mL; IGF1 16 ng/mL; TGFβ 2.3 ng/mL). Impaired angiogenic activity of 16p11.2df/+ of cECs was only partly rescued in these conditions. Data are mean ± s.e.m. in a and c, or whisker boxes (min to max, center line indicating median) in b (n = 4-5 animals per group). *P < 0.05 (2-way repeated measure ANOVA and Sidak’s post-hoc test).
Extended Data Fig. 9 Human iPSC lines used to derive endothelial cells, and the quality controls.
a, Representative images of cell morphology from culture steps (D=day) involved in differentiating human iPSC into human-induced endothelial cells (hiECs). Images are representative of 3 experiments repeated independently with similar results. b, Representative flow cytometric plots of MAC-selected CD144- positive cells from both control (healthy) and 16p11.2 individuals, demonstrating similarly high expression of endothelial markers CD31 and CD34. Conversely, CD144-negative hiECs show negligible expression of endothelial markers. Flow cytometric plots displayed are representative of 4 experiments repeated independently with similar results. c, Left, Sample images of immunocytochemical staining for endothelial marker VE-Cadherin in hiEC cultures. Right, Quantification of VE-Cadherin staining intensity across cell-cell junctions (total of 100 junctions/genotype) showing normal endothelial differentiation using 16p11.2 deletion iPSCs. d, Assessment of apoptotic rates in cell culture using a Caspase3/7 green assay shows no difference between control and 16p11.2 DEL hiECs. e, Assessment of proliferation in cell culture using an EdU incorporation assay shows no difference between control and 16p11.2 DEL hiECs. f, Quantification of core endothelium-enriched genes using ClariomTM S shows no differences between control and 16p11.2 DEL hiECs. g, Quantification of 16p11.2 locus genes using ClariomTM S microarray confirms hemizygosity of 16p11.2 DEL hiECs compared to control hiECs. DEL, deletion. Data are mean ± s.e.m. in c, f and g, whisker boxes (min to max, center line indicating median) in d, or mean with individual values in e (n = 3 cell lines per group). **P < 0.01, ***P < 0.001 (2-way ANOVA and Tukey’s post-hoc test).
Extended Data Fig. 10 Additional behavioral analysis of constitutive and conditional mutant mice and their controls.
a, b, Left, Assessment of home cage activity in the beam break test for combined 16p11.2ΔEC and control littermates (a), or combined male and female 16p11.2df/+ and WT mice (b). Right, First 12hrs of habituation (from testing day 1) in the beam break test for male and female 16p11.2ΔEC and control littermates (a), or 16p11.2df/+ and WT mice (b). c,d, Assessment of motor learning/coordination in the rotarod test for combined male and female 16p11.2df/+ and WT mice (c), or 16p11.2ΔEC and control littermates (d). e, The marble burying test revealed a phenotype for combined sexes in 16p11.2ΔEC mice (right), but not 16p11.2df/+ mice (left). f, The novel object recognition test revealed a phenotype for combined sexes in 16p11.2df/+ mice (left), but not for 16p11.2ΔEC mice (right). Data are mean ± s.e.m. in a-d, or mean with individual values in e and f (n = 9-18 mice per sex group). *P < 0.05, **P < 0.01, ***P < 0.001 (2-way repeated measure ANOVA and Sidak’s post-hoc test in a-c; Mann-Whitney test in e and f). ♂: males; ♀: females.
Supplementary information
Supplementary Information
Supplementary Figs. 1 and 2, and Supplementary Table 1.
Source data
Source Data Fig. 2
ANOVA tables.
Source Data Fig. 5
ANOVA tables.
Source Data Fig. 8
ANOVA tables.
Source Data Extended Data Fig. 2
ANOVA tables.
Source Data Extended Data Fig. 9
ANOVA tables.
Source Data Extended Data Fig. 10
ANOVA tables.
Rights and permissions
About this article
Cite this article
Ouellette, J., Toussay, X., Comin, C.H. et al. Vascular contributions to 16p11.2 deletion autism syndrome modeled in mice. Nat Neurosci 23, 1090–1101 (2020). https://doi.org/10.1038/s41593-020-0663-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-020-0663-1
This article is cited by
-
Sex, hormones and cerebrovascular function: from development to disorder
Fluids and Barriers of the CNS (2024)
-
The genetic landscape and clinical implication of pediatric Moyamoya angiopathy in an international cohort
European Journal of Human Genetics (2023)
-
Astroglial Hmgb1 regulates postnatal astrocyte morphogenesis and cerebrovascular maturation
Nature Communications (2023)
-
The amplitude of fNIRS hemodynamic response in the visual cortex unmasks autistic traits in typically developing children
Translational Psychiatry (2022)
-
Maternal high-fat diet in mice induces cerebrovascular, microglial and long-term behavioural alterations in offspring
Communications Biology (2022)