Abstract
The appropriate selection of passive and active defensive behaviors in threatening situations is essential for survival. Previous studies have shown that passive defensive responses depend on activity of the central nucleus of the amygdala (CeA), whereas active ones primarily rely on the nucleus accumbens (NAc). However, the mechanisms underlying flexible switching between these two types of responses remain unknown. Here we show in mice that the paraventricular thalamus (PVT) mediates the selection of defensive behaviors through its interaction with the CeA and the NAc. We show that the PVT–CeA pathway drives conditioned freezing responses, whereas the PVT–NAc pathway is inhibited during freezing and, instead, signals active avoidance events. Optogenetic manipulations revealed that activity in the PVT–CeA or PVT–NAc pathway biases behavior toward the selection of passive or active defensive responses, respectively. These findings provide evidence that the PVT mediates flexible switching between opposing defensive behaviors.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All the data that support the findings presented in this study are available from the corresponding author upon reasonable request. Source data are provided with this paper and are publicly available at the following repository: https://github.com/Penzolab/Source-Data-07092021.git.
Code availability
R code used to analyze active avoidance behavior and photometric signal is available at the following repository: https://github.com/Penzolab/Data-analysis-of-Two-way-active-avoidance-task.git.
References
LeDoux, J. E. Emotion circuits in the brain. Annu. Rev. Neurosci. 23, 155–184 (2000).
Duvarci, S. & Pare, D. Amygdala microcircuits controlling learned fear. Neuron 82, 966–980 (2014).
Fanselow, M. S. & Poulos, A. M. The neuroscience of mammalian associative learning. Annu. Rev. Psychol. 56, 207–234 (2005).
Fadok, J. P. et al. A competitive inhibitory circuit for selection of active and passive fear responses. Nature 542, 96–100 (2017).
LeDoux, J. E., Moscarello, J., Sears, R. & Campese, V. The birth, death and resurrection of avoidance: a reconceptualization of a troubled paradigm. Mol. Psychiatry 22, 24–36 (2017).
Cain, C. K. Avoidance problems reconsidered. Curr. Opin. Behav. Sci. 26, 9–17 (2019).
Krypotos, A. M., Effting, M., Kindt, M. & Beckers, T. Avoidance learning: a review of theoretical models and recent developments. Front. Behav. Neurosci. 9, 189 (2015).
Mobbs, D., Headley, D. B., Ding, W. & Dayan, P. Space, time, and fear: survival computations along defensive circuits. Trends Cogn. Sci. 24, 228–241 (2020).
Fanselow, M. S. & Lester, L. S. in Evolution and Learning 185–212 (Lawrence Erlbaum Associates, 1988).
Mobbs, D. The ethological deconstruction of fear(s). Curr. Opin. Behav. Sci. 24, 32–37 (2018).
Li, H. et al. Experience-dependent modification of a central amygdala fear circuit. Nat. Neurosci. 16, 332–339 (2013).
Penzo, M. A. et al. The paraventricular thalamus controls a central amygdala fear circuit. Nature 519, 455–459 (2015).
Bravo-Rivera, C., Roman-Ortiz, C., Brignoni-Perez, E., Sotres-Bayon, F. & Quirk, G. J. Neural structures mediating expression and extinction of platform-mediated avoidance. J. Neurosci. 34, 9736–9742 (2014).
Ramirez, F., Moscarello, J. M., LeDoux, J. E. & Sears, R. M. Active avoidance requires a serial basal amygdala to nucleus accumbens shell circuit. J. Neurosci. 35, 3470–3477 (2015).
Oleson, E. B., Gentry, R. N., Chioma, V. C. & Cheer, J. F. Subsecond dopamine release in the nucleus accumbens predicts conditioned punishment and its successful avoidance. J. Neurosci. 32, 14804–14808 (2012).
Choi, J. S., Cain, C. K. & LeDoux, J. E. The role of amygdala nuclei in the expression of auditory signaled two-way active avoidance in rats. Learn. Mem. 17, 139–147 (2010).
Moscarello, J. M. & LeDoux, J. E. Active avoidance learning requires prefrontal suppression of amygdala-mediated defensive reactions. J. Neurosci. 33, 3815–3823 (2013).
Beyeler, A. et al. Organization of valence-encoding and projection-defined neurons in the basolateral amygdala. Cell Rep. 22, 905–918 (2018).
Jimenez, S. A. & Maren, S. Nuclear disconnection within the amygdala reveals a direct pathway to fear. Learn. Mem. 16, 766–768 (2009).
Maren, S. Neurotoxic basolateral amygdala lesions impair learning and memory but not the performance of conditional fear in rats. J. Neurosci. 19, 8696–8703 (1999).
Poremba, A. & Gabriel, M. Amygdala neurons mediate acquisition but not maintenance of instrumental avoidance behavior in rabbits. J. Neurosci. 19, 9635–9641 (1999).
Do-Monte, F. H., Quinones-Laracuente, K. & Quirk, G. J. A temporal shift in the circuits mediating retrieval of fear memory. Nature 519, 460–463 (2015).
Vertes, R. P. & Hoover, W. B. Projections of the paraventricular and paratenial nuclei of the dorsal midline thalamus in the rat. J. Comp. Neurol. 508, 212–237 (2008).
Li, S. & Kirouac, G. J. Projections from the paraventricular nucleus of the thalamus to the forebrain, with special emphasis on the extended amygdala. J. Comp. Neurol. 506, 263–287 (2008).
Dong, X., Li, S. & Kirouac, G. J. Collateralization of projections from the paraventricular nucleus of the thalamus to the nucleus accumbens, bed nucleus of the stria terminalis, and central nucleus of the amygdala. Brain Struct. Funct. 222, 3927–3943 (2017).
Arcelli, P., Frassoni, C., Regondi, M. C., De Biasi, S. & Spreafico, R. GABAergic neurons in mammalian thalamus: a marker of thalamic complexity? Brain Res. Bull. 42, 27–37 (1997).
Fremeau, R. T. Jr., Voglmaier, S., Seal, R. P. & Edwards, R. H. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci. 27, 98–103 (2004).
Beas, B. S. et al. The locus coeruleus drives disinhibition in the midline thalamus via a dopaminergic mechanism. Nat. Neurosci. 21, 963–973 (2018).
Gao, C. et al. Two genetically, anatomically and functionally distinct cell types segregate across anteroposterior axis of paraventricular thalamus. Nat. Neurosci. 23, 217–228 (2020).
Clark, A. M. et al. Dopamine D2 receptors in the paraventricular thalamus attenuate cocaine locomotor sensitization. eNeuro 4, ENEURO.0227-17.2017 (2017)
McGinty, J. F. & Otis, J. M. Heterogeneity in the paraventricular thalamus: the traffic light of motivated behaviors. Front. Behav. Neurosci. 14, 590528 (2020).
McNally, G. P. Motivational competition and the paraventricular thalamus. Neurosci. Biobehav. Rev. 125, 193–207 (2021).
Li, Y., Dong, X., Li, S. & Kirouac, G. J. Lesions of the posterior paraventricular nucleus of the thalamus attenuate fear expression. Front. Behav. Neurosci. 8, 94 (2014).
Kirouac, G. J. Placing the paraventricular nucleus of the thalamus within the brain circuits that control behavior. Neurosci. Biobehav. Rev. 56, 315–329 (2015).
Choi, E. A. & McNally, G. P. Paraventricular thalamus balances danger and reward. J. Neurosci. 37, 3018–3029 (2017).
Penzo, M. A. & Gao, C. The paraventricular nucleus of the thalamus: an integrative node underlying homeostatic behavior. Trends Neurosci. 44, 538–549 (2021).
Watarai, A. et al. The blockade of oxytocin receptors in the paraventricular thalamus reduces maternal crouching behavior over pups in lactating mice. Neurosci. Lett. 720, 134761 (2020).
Zhu, Y., Wienecke, C. F., Nachtrab, G. & Chen, X. A thalamic input to the nucleus accumbens mediates opiate dependence. Nature 530, 219–222 (2016).
Meffre, J. et al. Orexin in the posterior paraventricular thalamus mediates hunger-related signals in the nucleus accumbens core. Curr. Biol. 29, 3298–3306 (2019).
Otis, J. M. et al. Paraventricular thalamus projection neurons integrate cortical and hypothalamic signals for cue-reward processing. Neuron 103, 423–431 (2019).
Labouebe, G., Boutrel, B., Tarussio, D. & Thorens, B. Glucose-responsive neurons of the paraventricular thalamus control sucrose-seeking behavior. Nat. Neurosci. 19, 999–1002 (2016).
Beas, B. S. et al. A ventrolateral medulla-midline thalamic circuit for hypoglycemic feeding. Nat. Commun. 11, 6218 (2020).
Ong, Z. Y., Liu, J. J., Pang, Z. P. & Grill, H. J. Paraventricular thalamic control of food intake and reward: role of glucagon-like peptide-1 receptor signaling. Neuropsychopharmacology 42, 2387–2397 (2017).
Keyes, P. C. et al. Orchestrating opiate-associated memories in thalamic circuits. Neuron 107, 1113–1123 (2020).
Namburi, P. et al. A circuit mechanism for differentiating positive and negative associations. Nature 520, 675–678 (2015).
Rogers-Carter, M. M., Djerdjaj, A., Gribbons, K. B., Varela, J. A. & Christianson, J. P. Insular cortex projections to nucleus accumbens core mediate social approach to stressed juvenile rats. J. Neurosci. 39, 8717–8729 (2019).
Schiff, H. C. et al. An insula–central amygdala circuit for guiding tastant-reinforced choice behavior. J. Neurosci. 38, 1418–1429 (2018).
Beier, K. T. et al. Circuit architecture of VTA dopamine neurons revealed by systematic input–output mapping. Cell 162, 622–634 (2015).
Heymann, G. et al. Synergy of distinct dopamine projection populations in behavioral reinforcement. Neuron 105, 909–920 (2020).
Kim, E. J., Juavinett, A. L., Kyubwa, E. M., Jacobs, M. W. & Callaway, E. M. Three types of cortical layer 5 neurons that differ in brain-wide connectivity and function. Neuron 88, 1253–1267 (2015).
Ren, J. et al. Anatomically defined and functionally distinct dorsal raphe serotonin sub-systems. Cell 175, 472–487 (2018).
Wallace, M. L. et al. Genetically distinct parallel pathways in the entopeduncular nucleus for limbic and sensorimotor output of the basal ganglia. Neuron 94, 138–152 (2017).
Laughlin, L. C., Moloney, D. M., Samels, S. B., Sears, R. M. & Cain, C. K. Reducing shock imminence eliminates poor avoidance in rats. Learn. Mem. 27, 270–274 (2020).
De Franceschi, G., Vivattanasarn, T., Saleem, A. B. & Solomon, S. G. Vision guides selection of freeze or flight defense strategies in mice. Curr. Biol. 26, 2150–2154 (2016).
Haynes, K., Fearnhead, P. & Eckley, I. A. A computationally efficient nonparametric approach for changepoint detection. Stat. Comput. 27, 1293–1305 (2017).
Acknowledgements
We thank former NIMH postbac N. Ringelberg for gathering preliminary findings that encouraged some aspects of the present study. In addition, we thank the NIMH IRP Rodent Behavioral Core for their support with the development of the custom apparatus for the 2AA task, the NIMH IRP Systems Neuroscience Imaging Resource for their support with the quantification of the rabies data and M. Hoon (NICHD) and F. Do Monte (UT Health) for offering scientific and writing feedback. This work was supported by the NIMH Intramural Research Program (1ZIAMH002950, to M.A.P.; MH002951 and MH002952, to Y.C.).
Author information
Authors and Affiliations
Contributions
J.M. performed all experiments. M.K. assisted with histological procedures and analyzed the monosynaptic rabies tracing data. B.S.B. assisted with the monosynaptic rabies tracing experiments. J.d.H. developed custom tools for analyzing behavior and calcium signals. J.M. and J.d.H. analyzed the data. Y.C. contributed to funding acquisition and writing. J.M. and M.A.P. designed the study, interpreted results and wrote the paper.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Peer review information Nature Neuroscience thanks Kay Tye and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 pPVTD2R neuron activity during fear conditioning and retrieval.
a, Representative image of GCaMP6s expression in pPVTD2R neurons and optical fiber placements (n = 6 mice). b, Experimental paradigm. c-e, Freezing behavior during the habituation (c), conditioning (d) and retrieval (e) sessions. f-h, Average calcium responses during the habituation (f), conditioning (g) and retrieval (h) sessions. i-k, Quantification of calcium signal during habituation (i), conditioning (j) and retrieval (k) sessions. AUC, One-way ANOVA followed by two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli. Habituation: n = 20 Trials; F(2, 57) = 0.1. Conditioning: n = 30 Trials; F(2, 87) = 2.17. Retrieval: n = 48 Trials; F(2, 141) = 7.7; group comparisons, pre-CS vs CS ***P = 0.0006, CS vs post-CS **P = 0.0012. l, Average calcium responses during early (Trials 1-3; left) and late (Trials 4-5; right) conditioning trials. m, Quantification of calcium signal during the first 5 s following the onset of CS during conditioning sessions. AUC, Two-way ANOVA followed by two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli, n = 12 Trials; F(4, 112) = 1.49. Group comparisons: Early, 1 s vs 4 s P = 0.054, 1 s vs 5 s *P = 0.021; Late, 1 s vs 3 s **P = 0.0043, 1 s vs 4 s ***P = 0.0003, 1 s vs 5 s ***P < 0.001, 2 s vs 5 s **P = 0.0035, 3 s vs 5 s *P = 0.049. n, Calcium signal during US presentation in the late trials is higher than the early trials (n = 6 mice; two-tailed paired Student’s t-test, P = 0.054). o, Top: Heatmaps showing calcium responses of Conditioning Trials 1-5 from individual subjects, respectively. Bottom: Average calcium responses of the top panels. All data in figure shown as mean ± s.e.m.
Extended Data Fig. 2 The activity of pPVTD2R neurons is positively correlated with movement during the CS following fear memory retrieval.
a, Calcium responses of individual retrieval trials aligned by percentage CS freezing (See Methods) (right). n = 6 mice, 8 trials per mouse. b, Left: Linear regression of CS calcium signal and freezing percentage for each trial. Right: Average CS calcium signal for each group (L, n = 20 trials; M, n = 16 trials; H, n = 12 trials). AUC, F(2, 45) = 3.3, one-way ANOVA followed by two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli. Group comparisons, L vs H, *P = 0.013. c, Average calcium responses (top), average movement index (middle) and linear regression of average calcium signal and movement index during the CS (bottom) for each group. d, Comparison of calcium signal (left) and movement index (right) for each group (L, n = 20 trials; M, n = 16 trials; H, n = 12 trials). AUC, two-way ANOVA followed by two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli. Calcium signal: F(4, 90) = 3.37; movement index: F(4, 90) = 3.6; for group comparisons ***P < 0.001, *P < 0.05. e-f, Average movement index (e) and linear regression of average calcium signal and movement index during the CS (f) for the habituation session. g, Individual subjects contributing to each group. h, Calcium responses of Trials 1-8 and average in bottom panels. i, Quantification of calcium signal during the Trials 1-8. AUC, One-way ANOVA followed by two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli. n = 6; Trial 1, F(2, 10) = 1.36; Trial 2, F(2, 10) = 4.55; Trial 3, F(2, 10) = 0.092; Trial 4, F(2, 10) = 1.55; Trial 5, F(2, 10) = 0.74; Trial 6, F(2, 10) = 2.83; Trial 7, F(2, 10) = 4.94; Trial 8, F(2, 10) = 2.71; for group comparisons *P < 0.05. j, Linear regression of average calcium signal and movement index from Trials 1-8. All data in figure shown as mean ± s.e.m.
Extended Data Fig. 3 The activity of pPVTD2R neurons in the 2AA task, related to Fig. 2.
a, Latency to avoid and freezing time during the ITI across days (n = 5 mice). b, Left: Linear regression of peak calcium signal and freezing time during the CS for avoidance (blue; A; R2 = 0.069, P = 0.0043) and failure trials (red; F; R2 = 0.15, P < 0.001). Right: Linear regression of average calcium signal and freezing time during the CS for avoidance (blue; A; R2 = 0.17, P < 0.001) and failure trials (red; F; R2 = 0.24, P < 0.001). c, Quantification of the latency to freezing after CS onset for avoidance and failure trials. Left: Counts of the freezing latency. Right: cumulative probability plots for the Left panel. Avoidance, n = 56 Events; Failure, n = 109 Events. d, Quantification of the latency to escape after CS onset for avoidance and failure trials. Left: Counts of the escape latency. Right: cumulative probability plots for the Left panel. Avoidance, n = 118; Failure, n = 122 Trials. All data in figure shown as mean ± s.e.m.
Extended Data Fig. 4 Optogenetic inhibition of pPVTD2R neurons in the 2AA task.
a, Representative image from a mouse expressing Halo-mCherry in pPVTD2R neurons and implanted with an optical fiber. b, Fiber placements (Ctl, n = 8 mice; Halo, n = 9 mice). c, Schematic of the 2AA task. d-g, Avoidance rate (d), latency to avoid (e) and freezing time during the CS (f) and the ITI (g) across training days for each group. h, Left: Freezing time during the ITI. Right: Normalization to Day 1 for each group. ITI freezing in s, two-way ANOVA followed by two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli. F(2, 30) = 0.36, Non-significant. i, Linear regression of the changes in freezing behavior across test sessions as a function of changes in avoidance behavior. j. Moving distance in the open field. Ctl, n = 8 mice; Halo, n = 5 mice. k, Schematic of the viral vector strategy and optical fiber placement used for optogenetic silencing of pPVTD2R neurons in the 2AA task. l, Fiber placements (Ctl, n = 6 mice; Halo, n = 7). m-p, Avoidance rate (m), latency to avoid (n) and freezing time during the CS (o) and the ITI (p) across training days in both Ctl and Halo groups. q-t, Left: Effect of optogenetic inhibition of pPVTD2Rneurons during the ITI on avoidance rate (q), the latency to avoid (r) and freezing time during the CS (s) and the ITI (t). Right: Normalization to Day 1 for each group. Two-way ANOVA followed by two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli. Avoidance rate, F(5, 80) = 9.05; latency to avoid, F(5, 80) = 4.52; CS freezing, F(5, 80) = 1.17; ITI freezing, F(5, 80) = 0.44; non-significant change among each group comparison. All data in figure shown as mean ± s.e.m.
Extended Data Fig. 5 Optogenetic inhibition of pPVT–NAc axon terminals in the 2AA task.
a, Schematic of the viral vector strategy and optical fiber placement for optogenetic silencing of pPVT–NAc axon terminals in the 2AA task. b, Fiber placements (Ctl, n = 13 mice; Halo, n = 11 mice). c-f, Avoidance rate (c), latency to avoid (d) and freezing time during the CS (e) and ITI (f) across training sessions for each group. g-j, Left: Avoidance rate (g), latency to avoid (h), freezing time during the CS (i) and the ITI (j) during optogenetic inhibition of pPVT–NAc axon terminals. Right: Normalization to Day 1 for each group. Two-way ANOVA followed by two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli. Avoidance rate: F(2, 44) = 4.89; group comparisons, Halo, Day 1 vs Day 2 *P = 0.013. Latency to avoid: F(2, 44) = 2.88; group comparisons, Halo, Day 1 vs Day 2 *P = 0.024. CS freezing: F(2, 44) = 1.1. ITI freezing: F(2, 44) = 0.46; non-significant change among other group comparison. k, Linear regression of the changes in freezing behavior across test sessions as a function of changes in avoidance behavior. l-o, Left: Optogenetic inhibition of pPVT–NAc axon terminals during the ITI has little effect on avoidance rate (l), the latency to avoid (m) and freezing time during the CS (n) and the ITI (o). Right: Normalization to Day 1 for each group. Ctl, n = 7 mice; Halo, n = 4 mice. Two-way ANOVA followed by two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli. Avoidance rate, F(2, 18) = 0.16; latency to avoid, F(2, 18) = 0.22; CS freezing, F(2, 18) = 0.037; ITI freezing, F(2, 18) = 0.48, Halo, Day 2 vs Day 3 *P = 0.022; non-significant change among other group comparison. p. Moving distance in the open field. Ctl, n = 9 mice; Halo, n = 8 mice. All data in figure shown as mean ± s.e.m.
Extended Data Fig. 6 Optogenetic inhibition of pPVT–CeA axon terminals in the 2AA task.
a, Schematic of the viral vector strategy and optical fiber placement for optogenetic silencing of pPVT–CeA axon terminals in the 2AA task. b, Fiber placements (Ctl, n = 11 mice; Halo, n = 12 mice). c-f, Avoidance rate (c), latency to avoid (d) and freezing time during the CS (e) and ITI (f) across training sessions for each group. g-j, Left: Avoidance rate (g), latency to avoid (h) and freezing time during the CS (i) and the ITI (j) of optogenetic inhibition of pPVT–CeA axon terminals. Right: Normalization to Day 1 for each group. Two-way ANOVA followed by two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli. Avoidance rate: F(2, 42) = 3.27. Latency to avoid: F(2, 42) = 5.35. CS freezing: F(2, 42) = 2.77. ITI freezing: F(2, 42) = 1.67. For group comparisons **P < 0.01, *P < 0.05. k, Linear regression of the changes in freezing behavior across test sessions as a function of changes in avoidance behavior. l-o, Left: Avoidance rate (l), the latency to avoid (m) and freezing time during the CS (n) and the ITI (o) during optogenetic inhibition of pPVT–CeA axon terminals during the ITI. Right: Normalization to Day 1 for each group. Ctl, n = 6 mice; Halo, n = 10 mice. Two-way ANOVA followed by two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli. Avoidance rate, F(2, 28) = 0.0052; latency to avoid, F(2, 28) = 0.22; CS freezing, F(2, 28) = 0.61; ITI freezing, F(2, 28) = 0.086; non-significant change among each group comparison. p. Moving distance in the open field. Ctl, n = 8 mice; Halo, n = 9 mice. All data in figure shown as mean ± s.e.m.
Extended Data Fig. 7 Optogenetic inhibition of pPVTD2R–CeA axon terminals in the 2AA task, related to Fig. 7.
a, Freezing time during the ITI across training days in both Ctl and Halo groups. n = 9 mice per group. b, Left: Optogenetic inhibition of pPVTD2R–CeA axon terminals gradually reduces freezing time during the the ITI. Right: Normalization to Day 1 for each group. Two-way ANOVA followed by two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli. F(5, 80) = 0.44; group comparisons, Ctl, Day 1 vs Day 6 ***P = 0.0006, Day 2 vs Day 6 *P = 0.011, Day 3 vs Day 6 **P = 0.0074, Day 4 vs Day 6 *P = 0.048; Halo, Day 1 vs Day 6 *P = 0.017. c, Linear regression of the changes in freezing behavior between Test Day 1 and other test sessions as a function of changes in avoidance behavior. All data in figure shown as mean ± s.e.m.
Extended Data Fig. 8 Optogenetic stimulation of pPVTD2R–NAc or pPVTD2R–CeA axon terminals in the 2AA task.
a, Schematic of the viral vector strategy and optical fiber placement for optogenetic stimulation of pPVTD2R–NAc axon terminals in the 2AA task. b, Fiber placements (Ctl, n = 8 mice; ChR, n = 12 mice). c-f, Avoidance rate (c), latency to avoid (d) and freezing time during the CS (e) and ITI (f) across training sessions for each group. g-j, Top: Avoidance rate (g), latency to avoid (h) and freezing time during the CS (i) and the ITI (j) during optogenetic stimulation of pPVTD2R–NAc axon terminals. Bottom: Normalization to Day 1 for each group. Two-way ANOVA followed by two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli. Avoidance rate: F(2, 36) = 6.21. Latency to avoid: F(2, 36) = 3.34. CS freezing: F(2, 36) = 0.16; ITI freezing: F(2, 36) = 0.21. For group comparisons **P < 0.01, *P < 0.05 k, Linear regression of the changes in freezing behavior across test sessions as a function of changes in avoidance behavior. l, Schematic of the viral vector strategy and optical fiber placement for optogenetic stimulating of pPVTD2R–CeA axon terminals in the 2AA task. m, Fiber placements (Ctl, n = 7 mice; ChR, n = 10 mice). n-q, Avoidance rate (n), latency to avoid (o) and freezing time during the CS (p) and ITI (q) across training sessions for both groups. r-u, Top: Avoidance rate (r), latency to avoid (s) and freezing time during the CS (t) and the ITI (u) during optogenetic stimulation of pPVTD2R–CeA axon terminals. Bottom: Normalization to Day 1 for each group. Two-way ANOVA followed by two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli. Avoidance rate: F(2, 30) = 5.43. Latency to avoid: F(2, 30) = 1.89. CS freezing: F(2, 30) = 0.88; ITI freezing: F(2, 30) = 0.66. For group comparisons **P < 0.01, *P < 0.05. v, Linear regression of the changes in freezing behavior across test sessions as a function of changes in avoidance behavior. All data in figure shown as mean ± s.e.m.
Extended Data Fig. 9 Optogenetic inhibition of pPVTD2R–NAc or pPVTD2R–CeA axon terminals in the 2AA task, related to Fig. 8.
a, Optical fiber placements (n = 8 mice per group). b, Freezing time during the ITI across training sessions for both Ctl and Halo groups. c, Left: Effect of optogenetic inhibition of pPVTD2R–NAc axon terminals (Day 2) and pPVTD2R–CeA axon terminals (Day 3) on ITI freezing. Right: Normalization to Day 1 for each group. Two-way ANOVA followed by two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli. F(3, 42) = 1.08; Halo, Day 1 vs Day 2 *P = 0.032. d, Linear regression of the changes in freezing behavior across test sessions as a function of changes in avoidance behavior. All data in figure shown as mean ± s.e.m.
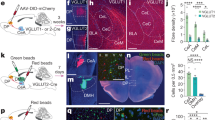
Extended Data Fig. 10 Monosynaptic inputs of NAc– and CeA–projecting neurons of the pPVT.
a, Schematic of the viral vector strategy to trace the inputs to NAc–projectors or CeA–projectors in pPVT. b, Representative images showing the rabies starter cells (Rabies-GFP and TVA-mCherry double-labelled cells) in pPVT neurons. c, Quantification of monosynaptic inputs to NAc–projectors or CeA–projectors in pPVT. NAc–projectors, n = 3 mice; CeA–projectors, n = 2 mice. To normalize retrogradely labeled (GFP+) cells between subjects, a connectivity index for each brain region was computed by dividing the number of retrogradely labeled cells by the number of starter cells (See Methods).
Supplementary information
Supplementary Video 1
Fiber photometry signal from pPVTD2R neurons from a sample subject. One exemplary avoidance trial and one exemplary failure trial are shown in the video.
Supplementary Video 2
Fiber photometry signal from pPVT–NAc neurons from a sample subject. One exemplary avoidance trial and one exemplary failure trial are shown in the video.
Source data
Source Data Fig. 1
Statistical Source Data
Source Data Fig. 2
Statistical Source Data
Source Data Fig. 3
Statistical Source Data
Source Data Fig. 4
Statistical Source Data
Source Data Fig. 5
Statistical Source Data
Source Data Fig. 6
Statistical Source Data
Source Data Fig. 7
Statistical Source Data
Source Data Fig. 8
Statistical Source Data
Source Data Extended Data Fig. 1
Statistical Source Data
Source Data Extended Data Fig. 2
Statistical Source Data
Source Data Extended Data Fig. 3
Statistical Source Data
Source Data Extended Data Fig. 4
Statistical Source Data
Source Data Extended Data Fig. 5
Statistical Source Data
Source Data Extended Data Fig. 6
Statistical Source Data
Source Data Extended Data Fig. 7
Statistical Source Data
Source Data Extended Data Fig. 8
Statistical Source Data
Source Data Extended Data Fig. 9
Statistical Source Data
Source Data Extended Data Fig. 10
Statistical Source Data
Rights and permissions
About this article
Cite this article
Ma, J., du Hoffmann, J., Kindel, M. et al. Divergent projections of the paraventricular nucleus of the thalamus mediate the selection of passive and active defensive behaviors. Nat Neurosci 24, 1429–1440 (2021). https://doi.org/10.1038/s41593-021-00912-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-021-00912-7
This article is cited by
-
Microglia govern the extinction of acute stress-induced anxiety-like behaviors in male mice
Nature Communications (2024)
-
Development and experience-dependent modulation of the defensive behaviors of mice to visual threats
The Journal of Physiological Sciences (2022)
-
The central nucleus of the amygdala and the construction of defensive modes across the threat-imminence continuum
Nature Neuroscience (2022)
-
Neurotensin orchestrates valence assignment in the amygdala
Nature (2022)