Abstract
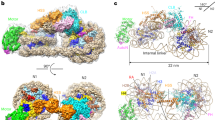
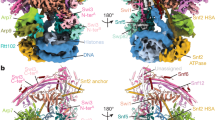
Access to chromatin for processes such as transcription and DNA repair requires the sliding of nucleosomes along DNA. This process is aided by chromatin-remodeling complexes, such as the multisubunit INO80 chromatin-remodeling complex. Here we present cryo-EM structures of the active core complex of human INO80 at 9.6 Å, with portions at 4.1-Å resolution, and reconstructions of combinations of subunits. Together, these structures reveal the architecture of the INO80 complex, including Ino80 and actin-related proteins, which is assembled around a single RUVBL1 (Tip49a) and RUVBL2 (Tip49b) AAA+ heterohexamer. An unusual spoked-wheel structural domain of the Ino80 subunit is engulfed by this heterohexamer; both, in combination, form the core of the complex. We also identify a cleft in RUVBL1 and RUVBL2, which forms a major interaction site for partner proteins and probably communicates these interactions to its nucleotide-binding sites.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Clapier, C. R. & Cairns, B. R. The biology of chromatin remodeling complexes. Annu. Rev. Biochem. 78, 273–304 (2009).
Singleton, M. R., Dillingham, M. S. & Wigley, D. B. Structure and mechanism of helicases and nucleic acid translocases. Annu. Rev. Biochem. 76, 23–50 (2007).
Delmas, V., Stokes, D. G. & Perry, R. P. A mammalian DNA-binding protein that contains a chromodomain and an SNF2/SWI2-like helicase domain. Proc. Natl. Acad. Sci. USA 90, 2414–2418 (1993).
Ito, T., Bulger, M., Pazin, M. J., Kobayashi, R. & Kadonaga, J. T. ACF, an ISWI-containing and ATP-utilizing chromatin assembly and remodeling factor. Cell 90, 145–155 (1997).
Cairns, B. R. et al. RSC, an essential, abundant chromatin-remodeling complex. Cell 87, 1249–1260 (1996).
Shen, X., Mizuguchi, G., Hamiche, A. & Wu, C. A chromatin remodelling complex involved in transcription and DNA processing. Nature 406, 541–544 (2000).
Jin, J. et al. A mammalian chromatin remodeling complex with similarities to the yeast INO80 complex. J. Biol. Chem. 280, 41207–41212 (2005).
Cai, Y. et al. Purification and assay of the human INO80 and SRCAP chromatin remodeling complexes. Methods 40, 312–317 (2006).
Chen, L. et al. Subunit organization of the human INO80 chromatin remodeling complex: an evolutionarily conserved core complex catalyzes ATP-dependent nucleosome remodeling. J. Biol. Chem. 286, 11283–11289 (2011).
Willhoft, O., Bythell-Douglas, R., McCormack, E. A. & Wigley, D. B. Synergy and antagonism in regulation of recombinant human INO80 chromatin remodeling complex. Nucleic Acids Res. 44, 8179–8188 (2016).
Erzberger, J. P. & Berger, J. M. Evolutionary relationships and structural mechanisms of AAA+ proteins. Annu. Rev. Biophys. Biomol. Struct. 35, 93–114 (2006).
Nano, N. & Houry, W. A. Chaperone-like activity of the AAA+ proteins Rvb1 and Rvb2 in the assembly of various complexes. Phil. Trans. R. Soc. Lond. B 368, 20110399 (2013).
Lakomek, K., Stoehr, G., Tosi, A., Schmailzl, M. & Hopfner, K. P. Structural basis for dodecameric assembly states and conformational plasticity of the full-length AAA+ ATPases Rvb1 • Rvb2. Structure 23, 483–495 (2015).
Silva-Martin, N. et al. The combination of x-ray crystallography and cryo-electron microscopy provides insight into the overall architecture of the Dodecameric Rvb1/Rvb2 complex. PLoS One 11, e0146457 (2016).
Ewens, C. A. et al. Architecture and nucleotide-dependent conformational changes of the Rvb1-Rvb2 AAA+ complex revealed by cryoelectron microscopy. Structure 24, 657–666 (2016).
Gorynia, S. et al. Structural and functional insights into a dodecameric molecular machine - the RuvBL1/RuvBL2 complex. J. Struct. Biol. 176, 279–291 (2011).
Matias, P. M., Gorynia, S., Donner, P. & Carrondo, M. A. Crystal structure of the human AAA+ protein RuvBL1. J. Biol. Chem. 281, 38918–38929 (2006).
Nguyen, V. Q. et al. Molecular architecture of the ATP-dependent chromatin-remodeling complex SWR1. Cell 154, 1220–1231 (2013).
Tosi, A. et al. Structure and subunit topology of the INO80 chromatin remodeler and its nucleosome complex. Cell 154, 1207–1219 (2013).
Willhoft, O. et al. Crosstalk within a functional INO80 complex dimer regulates nucleosome sliding. eLife 6, e25782 (2017).
Lin, C. L. et al. Functional characterization and architecture of recombinant yeast SWR1 histone exchange complex. Nucleic Acids Res. 45, 7249–7260 (2017).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Scheres, S. H. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012).
Shen, X., Ranallo, R., Choi, E. & Wu, C. Involvement of actin-related proteins in ATP-dependent chromatin remodeling. Mol. Cell 12, 147–155 (2003).
Szerlong, H. et al. The HSA domain binds nuclear actin-related proteins to regulate chromatin-remodeling ATPases. Nat. Struct. Mol. Biol. 15, 469–476 (2008).
Morrison, A. J. et al. INO80 and gamma-H2AX interaction links ATP-dependent chromatin remodeling to DNA damage repair. Cell 119, 767–775 (2004).
Watanabe, S. et al. Structural analyses of the chromatin remodelling enzymes INO80-C and SWR-C. Nat. Commun. 6, 7108 (2015).
Cao, T. et al. Crystal structure of a nuclear actin ternary complex. Proc. Natl. Acad. Sci. USA 113, 8985–8990 (2016).
Saravanan, M. et al. Interactions between the nucleosome histone core and Arp8 in the INO80 chromatin remodeling complex. Proc. Natl. Acad. Sci. USA 109, 20883–20888 (2012).
Chen, L., Conaway, R. C. & Conaway, J. W. Multiple modes of regulation of the human Ino80 SNF2 ATPase by subunits of the INO80 chromatin-remodeling complex. Proc. Natl. Acad. Sci. USA 110, 20497–20502 (2013).
Kelley, L. A., Mezulis, S., Yates, C. M., Wass, M. N. & Sternberg, M. J. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 10, 845–858 (2015).
Zhou, C. Y. et al. Regulation of Rvb1/Rvb2 by a domain within the INO80 chromatin remodeling complex implicates the yeast Rvbs as protein assembly chaperones. Cell Rep. 19, 2033–2044 (2017).
Yan, L., Wang, L., Tian, Y., Xia, X. & Chen, Z. Structure and regulation of the chromatin remodeller ISWI. Nature 540, 466–469 (2016).
Hauk, G., McKnight, J. N., Nodelman, I. M. & Bowman, G. D. The chromodomains of the Chd1 chromatin remodeler regulate DNA access to the ATPase motor. Mol. Cell 39, 711–723 (2010).
Zhang, D., Li, Y., Zhang, X., Zha, P. & Lin, R. The SWI2/SNF2 chromatin-remodeling ATPase BRAHMA regulates chlorophyll biosynthesis in Arabidopsis. Mol. Plant 10, 155–167 (2017).
Enemark, E. J., Chen, G., Vaughn, D. E., Stenlund, A. & Joshua-Tor, L. Crystal structure of the DNA binding domain of the replication initiation protein E1 from papillomavirus. Mol. Cell 6, 149–158 (2000).
Ripstein, Z. A., Huang, R., Augustyniak, R., Kay, L. E. & Rubinstein, J. L. Structure of a AAA+ unfoldase in the process of unfolding substrate. eLife 6, e25754 (2017).
Monroe, N., Han, H., Shen, P. S., Sundquist, W. I. & Hill, C. P. Structural basis of protein translocation by the Vps4-Vta1 AAA ATPase. eLife 6, e24487 (2017).
Yuan, Z. et al. Structural basis of Mcm2-7 replicative helicase loading by ORC-Cdc6 and Cdt1. Nat. Struct. Mol. Biol. 24, 316–324 (2017).
Schweitzer, A. et al. Structure of the human 26 S proteasome at a resolution of 3.9 Å. Proc. Natl. Acad. Sci. USA 113, 7816–7821 (2016).
Martin, A., Baker, T. A. & Sauer, R. T. Pore loops of the AAA+ ClpX machine grip substrates to drive translocation and unfolding. Nat. Struct. Mol. Biol. 15, 1147–1151 (2008).
Rappas, M. et al. Structural insights into the activity of enhancer-binding proteins. Science 307, 1972–1975 (2005).
Rappas, M., Schumacher, J., Niwa, H., Buck, M. & Zhang, X. Structural basis of the nucleotide driven conformational changes in the AAA+ domain of transcription activator PspF. J. Mol. Biol. 357, 481–492 (2006).
Lee, S. Y. et al. Regulation of the transcriptional activator NtrC1: structural studies of the regulatory and AAA+ ATPase domains. Genes Dev. 17, 2552–2563 (2003).
Glyde, R. et al. Structures of RNA polymerase closed and intermediate complexes reveal mechanisms of DNA opening and transcription initiation. Mol. Cell 67, 106–116.e4 (2017).
Zhang, X. & Wigley, D. B. The ‘glutamate switch’ provides a link between ATPase activity and ligand binding in AAA+ proteins. Nat. Struct. Mol. Biol. 15, 1223–1227 (2008).
Li, X. et al. Electron counting and beam-induced motion correction enable near-atomic-resolution single-particle cryo-EM. Nat. Methods 10, 584–590 (2013).
Rosenthal, P. B. & Henderson, R. Optimal determination of particle orientation, absolute hand, and contrast loss in single-particle electron cryomicroscopy. J. Mol. Biol. 333, 721–745 (2003).
Yang, Z., Fang, J., Chittuluru, J., Asturias, F. J. & Penczek, P. A. Iterative stable alignment and clustering of 2D transmission electron microscope images. Structure 20, 237–247 (2012).
Kiefer, F., Arnold, K., Künzli, M., Bordoli, L. & Schwede, T. The SWISS-MODEL Repository and associated resources. Nucleic Acids Res. 37, D387–D392 (2009).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Yang, J. et al. The I-TASSER Suite: protein structure and function prediction. Nat. Methods 12, 7–8 (2015).
Pettersen, E. F. et al. UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Krissinel, E. & Henrick, K. Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Crystallogr. D Biol. Crystallogr. 60, 2256–2268 (2004).
Winn, M. D. et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. D Biol. Crystallogr. 67, 235–242 (2011).
van Heel, M., Harauz, G., Orlova, E. V., Schmidt, R. & Schatz, M. A new generation of the IMAGIC image processing system. J. Struct. Biol. 116, 17–24 (1996).
Acknowledgements
We would like to thank D. Clare and A. Siebert at eBIC, Diamond Light Source, where the data were collected. We thank the members in the Section of Structural Biology for fruitful discussions. The work was funded by the Wellcome Trust Investigator awards 098412/Z/12/Z to X.Z. and 095519/Z/11/Z to D.B.W. and a Cancer Research UK grant C6913/A21608 to D.B.W.
Author information
Authors and Affiliations
Contributions
D.B.W. and X.Z. designed and supervised the studies. R.J.A. and R.A. performed the cryo-EM analysis. O.W. and R.B.-D. prepared and biochemically characterized the samples. R.B.-D., R.J.A. and O.W. built and refined the structural models. D.B.W. and X.Z. wrote the manuscript with input from all the authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated Supplementary Information
Supplementary Figure 1. Cryo-EM data processing of INO80 core complex
a. Typical micrograph, 2D classes, image processing schemes and angular distribution of particles. b. Gold-standard FSC curve calculated in RELION 1.4. c. Example of electron density regions and fitted model in RUVBL1 AAA+ domain and DII OB fold. d. Representative region in Ino80-I.
Supplementary Figure 2. Image processing and reconstructions of INO80 core, SC2 and SC2plus
a. Cryo-EM micrographs, 2D classes and 3D reconstructions. b. gold-standard FSC curves of the three reconstructions calculated in cryoSPARC with a mask calculated from the map, extended by 4 pixels and a soft edge of 4-7 pixels.
Supplementary Figure 3. Arp5–Ies6 assignment in the reconstructions
Overlay of the tail density of hINO80 sub-complex (solid) and hINO80 core complex (mesh) in two orthogonal views. An actin model is fitted into the hINO80 sub-complex density.
Supplementary Figure 4. RUVBL1 and RUVBL2 nucleotide-binding pockets
RUVBL1 chains are colored in blue and RUVBL2 chains are colored in cyan. Walker A Lys, Walker B Asp/Glu, Sensor I residue Asn as well as Arg fingers are labeled. Grey mesh is the 4.1 Å map. Red mesh is the difference map that corresponds to the 4.1 Å map with density of RUVBL1–2 being subtracted. The difference map shows clear density for ADP in all subunits while there is no density for γ-phosphate of ATP.
Supplementary Figure 5. Comparisons of RUVBL1 and RUVBL2 in the INO80 complex
a. Comparisons of the monomers, left panels: RUVBL1 monomers (chains A, C and E), right panel: RUVBL2 monomers (B, D and F). b. Comparisons of the dimers, left panels: RUVBL1–RUVBL2 dimer pairs, right panels: RUVBL2–RUVBL1 dimer pairs
Supplementary Figure 6. Partner binding clefts in RUVBL1 and RUVBL2
(a-c) shows the surface potential of the binding cleft formed by RUVBL1 DII helical bundle in the INO80 core complex and Ino80-I structure that it binds to. (d-f) same as (a-c) but with RUVBL2. RUVBL1 and RUVBL2 both have a hydrophobic core but RUVBL1 is more positively charged while RUVBL2 is more negatively charged adjacent to the core. Furthermore, each cleft conformation differs slightly to further enhance the structural plasticity.
Supplementary Figure 7. Comparisons of binding clefts of RUVBL1 and RUVBL2 in hINO80 with those of PDB 4WW4
(a) Comparison of hRUVBL2 in the hINO80 core with ctRUVBL2 showing the differences in the DII cleft. (b) OB fold of ctRUVBL1 occupies the space of Ino80-I. (c) Superposition of hRUVBL1 with ctRUVBL1. (d) The N-terminus of ctRUVBL1 tucks into the DII binding cleft of ctRUVBL1.
Supplementary information
Supplementary Figures 1–7 and Table 1
Supplementary Figures 1–7 and Relative rotation (in degrees) of OB domains between protomers when main chains are aligned.
Supplementary Note 1
Sequence alignments of human, yeast, mouse, and C. thermophilum RUVBL1 and RUVBL2. Secondary structures, functional motifs and domains are labeled.
Rights and permissions
About this article
Cite this article
Aramayo, R.J., Willhoft, O., Ayala, R. et al. Cryo-EM structures of the human INO80 chromatin-remodeling complex. Nat Struct Mol Biol 25, 37–44 (2018). https://doi.org/10.1038/s41594-017-0003-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-017-0003-7
This article is cited by
-
The multi-faceted roles of R2TP complex span across regulation of gene expression, translation, and protein functional assembly
Biophysical Reviews (2023)
-
RUVBL1 promotes enzalutamide resistance of prostate tumors through the PLXNA1-CRAF-MAPK pathway
Oncogene (2022)
-
The nuclear actin-containing Arp8 module is a linker DNA sensor driving INO80 chromatin remodeling
Nature Structural & Molecular Biology (2018)
-
RPAP3 provides a flexible scaffold for coupling HSP90 to the human R2TP co-chaperone complex
Nature Communications (2018)
-
Structural basis for ATP-dependent chromatin remodelling by the INO80 complex
Nature (2018)