Abstract
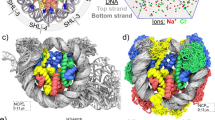
Nucleosomes, the basic units of chromatin, package and regulate expression of eukaryotic genomes. Although the structure of the intact nucleosome is well characterized, little is known about structures of partially unwrapped, transient intermediates. In this study, we present nine cryo-EM structures of distinct conformations of nucleosome and subnucleosome particles. These structures show that initial DNA breathing induces conformational changes in the histone octamer, particularly in histone H3, that propagate through the nucleosome and prevent symmetrical DNA opening. Rearrangements in the H2A–H2B dimer strengthen interaction with the unwrapping DNA and promote nucleosome stability. In agreement with this, cross-linked H2A–H2B that cannot accommodate unwrapping of the DNA is not stably maintained in the nucleosome. H2A–H2B release and DNA unwrapping occur simultaneously, indicating that DNA is essential in stabilizing the dimer in the nucleosome. Our structures reveal intrinsic nucleosomal plasticity that is required for nucleosome stability and might be exploited by extrinsic protein factors.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Cutter, A. R. & Hayes, J. J. A brief review of nucleosome structure. FEBS Lett. 589(20 Pt A), 2914–2922 (2015).
Andrews, A. J. & Luger, K. Nucleosome structure(s) and stability: variations on a theme. Annu. Rev. Biophys. 40, 99–117 (2011).
Luger, K., Mäder, A. W., Richmond, R. K., Sargent, D. F. & Richmond, T. J. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature 389, 251–260 (1997).
Luger, K., Dechassa, M. L. & Tremethick, D. J. New insights into nucleosome and chromatin structure: an ordered state or a disordered affair? Nat. Rev. Mol. Cell Biol. 13, 436–447 (2012).
Li, G., Levitus, M., Bustamante, C. & Widom, J. Rapid spontaneous accessibility of nucleosomal DNA. Nat. Struct. Mol. Biol. 12, 46–53 (2005).
Miyagi, A., Ando, T. & Lyubchenko, Y. L. Dynamics of nucleosomes assessed with time-lapse high-speed atomic force microscopy. Biochemistry 50, 7901–7908 (2011).
Lyubchenko, Y. L. Nanoscale nucleosome dynamics assessed with time-lapse AFM. Biophys. Rev. 6, 181–190 (2014).
Ngo, T. T. M. & Ha, T. Nucleosomes undergo slow spontaneous gaping. Nucleic Acids Res. 43, 3964–3971 (2015).
Li, G. & Widom, J. Nucleosomes facilitate their own invasion. Nat. Struct. Mol. Biol. 11, 763–769 (2004).
Hodges, C., Bintu, L., Lubkowska, L., Kashlev, M. & Bustamante, C. Nucleosomal fluctuations govern the transcription dynamics of RNA polymerase II. Science 325, 626–628 (2009).
Rhee, H. S., Bataille, A. R., Zhang, L. & Pugh, B. F. Subnucleosomal structures and nucleosome asymmetry across a genome. Cell 159, 1377–1388 (2014).
Sheinin, M. Y., Li, M., Soltani, M., Luger, K. & Wang, M. D. Torque modulates nucleosome stability and facilitates H2A/H2B dimer loss. Nat. Commun. 4, 2579 (2013).
Bintu, L. et al. The elongation rate of RNA polymerase determines the fate of transcribed nucleosomes. Nat. Struct. Mol. Biol. 18, 1394–1399 (2011).
Kireeva, M. L. et al. Nucleosome remodeling induced by RNA polymerase II: loss of the H2A/H2B dimer during transcription. Mol. Cell 9, 541–552 (2002).
Engeholm, M. et al. Nucleosomes can invade DNA territories occupied by their neighbors. Nat. Struct. Mol. Biol. 16, 151–158 (2009).
Kato, D. et al. Crystal structure of the overlapping dinucleosome composed of hexasome and octasome. Science 356, 205–208 (2017).
Böhm, V. et al. Nucleosome accessibility governed by the dimer/tetramer interface. Nucleic Acids Res. 39, 3093–3102 (2011).
Chen, Y. et al. Asymmetric unwrapping of nucleosomal DNA propagates asymmetric opening and dissociation of the histone core. Proc. Natl. Acad. Sci. USA 114, 334–339 (2017).
Ngo, T. T. M., Zhang, Q., Zhou, R., Yodh, J. G. & Ha, T. Asymmetric unwrapping of nucleosomes under tension directed by DNA local flexibility. Cell 160, 1135–1144 (2015).
Lowary, P. T. & Widom, J. New DNA sequence rules for high affinity binding to histone octamer and sequence-directed nucleosome positioning. J. Mol. Biol. 276, 19–42 (1998).
Ivić, N., Groschup, B., Bilokapić, S. & Halić, M. Simplified method for rapid purification of soluble histones. Croat. Chem. Acta 89, 153–162 (2016).
Zocco, M., Marasovic, M., Pisacane, P., Bilokapic, S. & Halic, M. The Chp1 chromodomain binds the H3K9me tail and the nucleosome core to assemble heterochromatin. Cell Discov. 2, 16004 (2016).
Chua, E. Y. D. et al. 3.9 Å structure of the nucleosome core particle determined by phase-plate cryo-EM. Nucleic Acids Res. 44, 8013–8019 (2016).
Chen, Y. et al. Revealing transient structures of nucleosomes as DNA unwinds. Nucleic Acids Res. 42, 8767–8776 (2014).
Vasudevan, D., Chua, E. Y. D. & Davey, C. A. Crystal structures of nucleosome core particles containing the ‘601’ strong positioning sequence. J. Mol. Biol. 403, 1–10 (2010).
McGinty, R. K. & Tan, S. Nucleosome structure and function. Chem. Rev. 115, 2255–2273 (2015).
Iwasaki, W. et al. Contribution of histone N-terminal tails to the structure and stability of nucleosomes. FEBS Open Bio 3, 363–369 (2013).
Liu, X., Li, M., Xia, X., Li, X. & Chen, Z. Mechanism of chromatin remodelling revealed by the Snf2-nucleosome structure. Nature 544, 440–445 (2017).
Tachiwana, H. et al. Crystal structure of the human centromeric nucleosome containing CENP-A. Nature 476, 232–235 (2011).
Arimura, Y., Tachiwana, H., Oda, T., Sato, M. & Kurumizaka, H. Structural analysis of the hexasome, lacking one histone H2A/H2B dimer from the conventional nucleosome. Biochemistry 51, 3302–3309 (2012).
Park, Y.-J., Chodaparambil, J. V., Bao, Y., McBryant, S. J. & Luger, K. Nucleosome assembly protein 1 exchanges histone H2A-H2B dimers and assists nucleosome sliding. J. Biol. Chem. 280, 1817–1825 (2005).
Hall, M. A. et al. High-resolution dynamic mapping of histone-DNA interactions in a nucleosome. Nat. Struct. Mol. Biol. 16, 124–129 (2009).
Anderson, M. et al. Co-expression as a convenient method for the production and purification of core histones in bacteria. Protein Expr. Purif. 72, 194–204 (2010).
Shim, Y., Duan, M.-R., Chen, X., Smerdon, M. J. & Min, J.-H. Polycistronic coexpression and nondenaturing purification of histone octamers. Anal. Biochem. 427, 190–192 (2012).
Luger, K., Rechsteiner, T. J. & Richmond, T. J. Expression and purification of recombinant histones and nucleosome reconstitution. Methods Mol. Biol. 119, 1–16 (1999).
Grant, T. & Grigorieff, N. Measuring the optimal exposure for single particle cryo-EM using a 2.6 Å reconstruction of rotavirus VP6. eLife 4, e06980 (2015).
Scheres, S. H. W., Núñez-Ramírez, R., Sorzano, C. O. S., Carazo, J. M. & Marabini, R. Image processing for electron microscopy single-particle analysis using XMIPP. Nat. Protoc. 3, 977–990 (2008).
Rohou, A. & Grigorieff, N. CTFFIND4: Fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Scheres, S. H. W. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Pettersen, E. F. et al. UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Acknowledgements
We would like to thank E. Conti and the Cryo-EM facility at Max Planck Institute for Biochemistry in Martinsried for access to cryo-EM microscopes. Without their support, this work would not have been possible. We also acknowledge the EMBO YIP small grant for supporting the data collection at NECEN in Leiden. The authors would like to thank R. Büchner and H. Thomas for help with the histone purification and S. Jaklin for excellent technical assistance. We also thank the Halic lab for comments on the manuscript. This work was supported by the ERC-smallRNAhet-309584 and the Instruct PID 1498.
Author information
Authors and Affiliations
Contributions
S.B. and M.H. designed the experiments. S.B. performed biochemical experiments and EM. M.S. assisted with EM. S.B. and M.H. analyzed the data. S.B. and M.H. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 Cryo-EM reconstruction of NCP (class 1).
a, Native gel showing nucleosome assembly. Uncropped gel image is shown in Supplementary Data Set 1. b, Representative cryo-EM raw micrograph of dataset A collected with Titan Halo electron microscope at 300 keV. NCPs in multiple orientations are clearly visible. c, Representative 2D class averages showing nucleosome core particles in different orientations (side, tilted and top views). Many details are visible in 2D class averages. d, Cryo-EM map of the NCP class 1 structure at 3.7 Å (0.143 cutoff in FSC curve). e, Fourier shell correlation (FSC) curve showing the resolution of the class 1 cryo-EM map (blue). The red curve shows FSC between the final refined atomic model and the combined map that the model was refined against. The purple curves shows FSC between the atomic model and the half map it was refined against (half 1). The dark red curve shows FSC between the atomic model and the other half map it was not refined against (half 2). f, Molecular model fitted to the class 1 cryo-EM map showing that side chains are resolved in the histone core. g, Class 1 cryo-EM structure shows an appearance of a canonical nucleosome. Comparison of class 1 (blue) and X-ray PDB:3LZ1 (yellow) models.
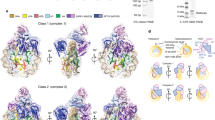
Supplementary Figure 2 Cryo-EM reconstructions of NCP (classes 2–4).
a, Cryo-EM maps of class 2 (purple), class 3 (pink) and class 4 (red) NCPs at 5.4 Å, 5.1 Å and 6.4 Å respectively. b, Fourier shell correlation (FSC) curve showing the resolution of class 2-4 cryo-EM maps (0.143 cutoff in FSC curve) (blue line). The red curve shows FSC between the final refined atomic model and the combined map that the model was refined against. The purple curves shows FSC between the atomic model and the half map it was refined against (half 1). The dark red curve shows FSC between the atomic model and the other half map it was not refined against (half 2). c, Local resolution estimate in Relion showing local resolution of class 1–4 cryo-EM maps.
Supplementary Figure 3 X-ray model refined into cryo-EM maps.
a, Overall fit of the class 2 (purple), class 3 (pink) and class 4 (red) models into the corresponding cryo-EM maps. b, Fitting of the class 2 (purple), class 3 (pink) and class 4 (red) models into the corresponding cryo-EM maps. The H3 α1, α2, α3 and H4 α2 from all three models fit well into corresponding cryo-EM maps. Nucleosome half with unwrapped DNA is shown. c, Fitting of the class 2 (purple), class 3 (pink) and class 4 (red) models into the corresponding cryo-EM maps. The H3 αN and H2A–H2B from all three models fit well into corresponding cryo-EM maps. Nucleosome half with wrapped DNA is shown. d, Global changes in the nucleosome structure in class 2–4 cryo-EM maps. Comparison of class 2 (purple), class 3 (pink) and class 4 (red). In class 4 nucleosome expands in the direction perpendicular to the symmetry axis (distance between C3 in nucleotides 19 and −19) and contracts along the symmetry axis (distance between C3 in nucleotides 2 and 38).
Supplementary Figure 4 Histone H3 and H4 rearrange as DNA unwraps.
a, Model for the class 2 cryoEM map is displayed for orientation purposes. On the unwrapped half H3–H4 is colored ligh gray and H2A–H2B dark gray. On the wrapped half, H3–H4 is colored dark blue and H2A–H2B light blue. b, Models for the class 1 (blue) and the class 4 (red) cryo-EM maps are overlayed with the best overall fit. The orientation is the same like in Fig. 2a. Changes are more prominent on the unwrapped half. On the wrapped half, H2A–H2B and H4 do not rearrange. c, Conformational rearrangement of H3 in class 4 (red) structure near the dyad. H3 α2 on unwrapped and wrapped half move when compared to the lcass 1 model (blue). DNA at the dyad moves toward the center of the nucleosome. d, Conformational rearrangement of H4 in class 4 (red) structure near the dyad. uH4 α1 and uH4 α2 move when compared to the class 1 model (blue). DNA at the SHL −2 moves outward. wH4 α1 also moves but wH4 α2/3 on the wrapped side of the nucleosome did not rearrange. e, DNA unwrapping leads to conformational changes in H3 that propagate through the nucleosome. DNA at the second entry–exit-site is moved toward the H3 αN and the dyad. This rearrangement of the nucleosome might stabilize the DNA at the second entry–exit-site to prevent symmetrical opening.
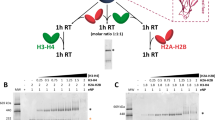
Supplementary Figure 5 Crosslinked (CL) H2A–H2B is not stably bound to the nucleosome.
a, Chromatogram and SDS-PAGE showing assembly of the native octamer. Excess of H2A–H2B dimer, used in the octamer assembly, was removed by size exclusion chromatography (black line). Red line shows migration of the H2A–H2B dimer. Selected fractions of assembled octamer, marked with a black bar on the chromatogram, were analyzed by SDS-PAGE. b, Chromatogram and SDS-PAGE showing assembly of the octamer with crosslinked H2A–H2B. Excess of crosslinked H2A–H2B dimer, used in the octamer assembly, was removed by size exclusion chromatography (blue line). Orange line shows migration of the crosslinked H2A–H2B dimer. Selected fractions from the octamer assembly, marked with a blue bar on the chromatogram, were analyzed by SDS-PAGE. c, Native gel showing Nap1 binding to native and crosslinked H2A–H2B. d, Native gel showing Nap1 binding to native and crosslinked globular H2A–H2B. e, SDS-PAGE showing migration of native and crosslinked full length and globular H2A–H2B. Representative images of at least 3 independent experiments are shown. Uncropped gel images are shown in Supplementary Data Set 1.
Supplementary Figure 6 Cryo-EM reconstructions of NCP (dataset B).
a, Native gel showing nucleosome assembly. Uncropped gel image is shown in Supplementary Data Set 1. b, Representative cryo-EM raw micrograph of dataset B collected with Titan Halo electron microscope at 300 keV. NCPs in multiple orientations are clearly visible. Free DNA is also visible in this data set. c, Representative 2D class averages showing nucleosome core particles in different orientations (side, tilted and top views). In many 2D class averages DNA protruding from NCP is visible. d, Cryo-EM map of NCP (dataset B) at 9.2 Å (0.143 cutoff in FSC curve). e, Fourier shell correlation (FSC) curve showing the resolution of cryo-EM map B.
Supplementary Figure 7 Classification of dataset B.
Five classes (class 5–9) of distinct conformations of NCPs and hexasomes were obtained. Fourier shell correlation (FSC) curves showing the resolution of cryo-EM maps of classes 5–9 are shown next to maps (0.143 cutoff in FSC curve).
Supplementary Figure 8 Model docking and local resolution calculation for classes 5–9.
(a, b, c, d, e) X-ray model of NCP (PDB ID 3LZ1, yellow) was fitted in maps of classes 5–9 (top). Parts of NCP not visible in classes 5–9 are shown in red. In c partially delocalized H2A–H2B is shown in orange. Local resolution estimate for classes 5–9 shows that the protein density is better resolved as compared to the DNA density (middle and bottom panels). Density for H2A–H2B is already less resolved in classes 5 and 6, poorly resolved in class 7 and not present in classes 8 and 9. On the side 2 the H2A–H2B density is present in each class and it is present at similar resolution as the remaining histones in the protein core.
Supplementary Figure 9 Model validation for class 1 and class 4.
a R.m.s.d. (Cα) between the half1 and half2 models for the class 1 and the class 4 half models. Deviation between two models from half data sets is less than 0.2 A for most regions in class 1 map. Deviation between two models from half data sets is less than 1.0 A for most regions in class 4 map. For better comparison, the resolution range is the same as in the Fig. 2a where we compare class 1 and class 4 models. b View at H2A–H2B near SHL5 and H3 α1 on the unwrapped side of the nucleosome. Class 1 model (blue) is superimposed with class 4 cryo-EM map (left). Class 1 cryo-EM map (light blue) is superimposed with class 4 cryo-EM map (center). On the right side the difference map (green) between class 4 and class 1 cryo-EM maps is shown.
Supplementary information
Supplementary Figures
Supplementary Figures 1–9
Supplementary Dataset 1
Uncropped gel images
Rights and permissions
About this article
Cite this article
Bilokapic, S., Strauss, M. & Halic, M. Histone octamer rearranges to adapt to DNA unwrapping. Nat Struct Mol Biol 25, 101–108 (2018). https://doi.org/10.1038/s41594-017-0005-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-017-0005-5
This article is cited by
-
Functionalized graphene-oxide grids enable high-resolution cryo-EM structures of the SNF2h-nucleosome complex without crosslinking
Nature Communications (2024)
-
Histone variant H2A.Z modulates nucleosome dynamics to promote DNA accessibility
Nature Communications (2023)
-
Structure of native chromatin fibres revealed by Cryo-ET in situ
Nature Communications (2023)
-
Histone modifications regulate pioneer transcription factor cooperativity
Nature (2023)
-
Are extraordinary nucleosome structures more ordinary than we thought?
Chromosoma (2023)