Abstract
Thermostable cross-β structures are characteristic of pathological amyloid fibrils, but these structures cannot explain the reversible nature of fibrils formed by RNA-binding proteins such as fused in sarcoma (FUS), involved in RNA granule assembly. Here, we find that two tandem (S/G)Y(S/G) motifs of the human FUS low-complexity domain (FUS LC) form reversible fibrils in a temperature- and phosphorylation-dependent manner. We named these motifs reversible amyloid cores, or RAC1 and RAC2, and determined their atomic structures in fibrillar forms, using microelectron and X-ray diffraction techniques. The RAC1 structure features an ordered-coil fibril spine rather than the extended β-strand typical of amyloids. Ser42, a phosphorylation site of FUS, is critical in the maintenance of the ordered-coil structure, which explains how phosphorylation controls fibril formation. The RAC2 structure shows a labile fibril spine with a wet interface. These structures illuminate the mechanism of reversible fibril formation and dynamic assembly of RNA granules.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
22 May 2018
In the HTML version of this article originally published, the delta symbols in the legend for Fig. 1d appeared as question marks for the following text: ΔRAC1, ΔRAC2 and ΔRAC1 + ΔRAC2. The errors have been corrected in the online version of the article.
References
Knowles, T. P., Vendruscolo, M. & Dobson, C. M. The amyloid state and its association with protein misfolding diseases. Nat. Rev. Mol. Cell Biol. 15, 384–396 (2014).
Eisenberg, D. & Jucker, M. The amyloid state of proteins in human diseases. Cell 148, 1188–1203 (2012).
Riek, R. & Eisenberg, D. S. The activities of amyloids from a structural perspective. Nature 539, 227–235 (2016).
Eisenberg, D. S. & Sawaya, M. R. Structural studies of amyloid proteins at the molecular level. Annu. Rev. Biochem. 86, 69–95 (2017).
Nelson, R. et al. Structure of the cross-beta spine of amyloid-like fibrils. Nature 435, 773–778 (2005).
Sun, Z. et al. Molecular determinants and genetic modifiers of aggregation and toxicity for the ALS disease protein FUS/TLS. PLoS Biol. 9, e1000614 (2011).
Kato, M. et al. Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels. Cell 149, 753–767 (2012).
Murakami, T. et al. ALS/FTD mutation-induced phase transition of fus liquid droplets and reversible hydrogels into irreversible hydrogels impairs rnp granule function. Neuron 88, 678–690 (2015).
Han, T. W. et al. Cell-free formation of RNA granules: bound RNAs identify features and components of cellular assemblies. Cell 149, 768–779 (2012).
Taylor, J. P., Brown, R. H. Jr. & Cleveland, D. W. Decoding ALS: from genes to mechanism. Nature 539, 197–206 (2016).
Aguzzi, A. & Altmeyer, M. Phase separation: linking cellular compartmentalization to disease. Trends Cell Biol. 26, 547–558 (2016).
Li, Y. R., King, O. D., Shorter, J. & Gitler, A. D. Stress granules as crucibles of ALS pathogenesis. J. Cell Biol. 201, 361–372 (2013).
Ramaswami, M., Taylor, J. P. & Parker, R. Altered ribostasis: RNA-protein granules in degenerative disorders. Cell 154, 727–736 (2013).
Anderson, P. & Kedersha, N. RNA granules: post-transcriptional and epigenetic modulators of gene expression. Nat. Rev. Mol. Cell Biol. 10, 430–436 (2009).
Shukla, S. & Parker, R. Hypo- and hyper-assembly diseases of RNA-protein complexes. Trends Mol. Med. 22, 615–628 (2016).
Zheng, D. et al. Deadenylation is prerequisite for P-body formation and mRNA decay in mammalian cells. J. Cell Biol. 182, 89–101 (2008).
Wu, H. & Fuxreiter, M. The structure and dynamics of higher-order assemblies: amyloids, signalosomes, and granules. Cell 165, 1055–1066 (2016).
Protter, D. S. & Parker, R. Principles and properties of stress granules. Trends Cell Biol. 26, 668–679 (2016).
Schuller, R. & Eick, D. Getting access to low-complexity domain modifications. Trends Biochem. Sci. 41, 894–897 (2016).
Kwiatkowski, T. J. Jr. et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science 323, 1205–1208 (2009).
Gitler, A. D. & Shorter, J. RNA-binding proteins with prion-like domains in ALS and FTLD-U. Prion 5, 179–187 (2011).
Patel, A. et al. A liquid-to-solid phase transition of the als protein fus accelerated by disease mutation. Cell 162, 1066–1077 (2015).
Gardiner, M., Toth, R., Vandermoere, F., Morrice, N. A. & Rouse, J. Identification and characterization of FUS/TLS as a new target of ATM. Biochem. J. 415, 297–307 (2008).
Deng, Q. et al. FUS is phosphorylated by DNA-PK and accumulates in the cytoplasm after DNA damage. J. Neurosci. 34, 7802–7813 (2014).
Kwon, I. et al. Phosphorylation-regulated binding of RNA polymerase II to fibrous polymers of low-complexity domains. Cell 155, 1049–1060 (2013).
Miguel, L. et al. Accumulation of insoluble forms of FUS protein correlates with toxicity in Drosophila. Neurobiol. Aging 33, 1008.e1–1008.e15 (2012).
Sawaya, M. R. et al. Atomic structures of amyloid cross-beta spines reveal varied steric zippers. Nature 447, 453–457 (2007).
Li, D. et al. Designed amyloid fibers as materials for selective carbon dioxide capture. Proc. Natl Acad. Sci. USA 111, 191–196 (2014).
Molliex, A. et al. Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell 163, 123–133 (2015).
de la Cruz, M. J. et al. Atomic-resolution structures from fragmented protein crystals with the cryoEM method MicroED. Nat. Methods 14, 399–402 (2017).
Tayeb-Fligelman, E. et al. The cytotoxic Staphylococcus aureus PSMα3 reveals a cross-α amyloid-like fibril. Science 355, 831–833 (2017).
Belzil, V. V. et al. Mutations in FUS cause FALS and SALS in French and French Canadian populations. Neurology 73, 1176–1179 (2009).
Monahan, Z. et al. Phosphorylation of the FUS low-complexity domain disrupts phase separation, aggregation, and toxicity. EMBO J. 36, 2951–2967 (2017).
March, Z. M., King, O. D. & Shorter, J. Prion-like domains as epigenetic regulators, scaffolds for subcellular organization, and drivers of neurodegenerative disease. Brain Res. 1647, 9–18 (2016).
Li, D. et al. Structure-based design of functional amyloid materials. J. Am. Chem. Soc. 136, 18044–18051 (2014).
Hughes, M. P. et al. Atomic structures of low-complexity protein segments reveal kinked β sheets that assemble networks. Science 359, 698–701 (2018).
Murray, D. T. et al. Structure of FUS protein fibrils and its relevance to self-assembly and phase separation of low-complexity domains. Cell 171, 615–627.e16 (2017).
Li, P. et al. Phase transitions in the assembly of multivalent signalling proteins. Nature 483, 336–340 (2012).
Kabsch, W. XDS. Acta Crystallogr. D Biol. Crystallogr 66, 125–132 (2010).
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Afonine, P. V. et al. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. D Biol. Crystallogr. 68, 352–367 (2012).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Acknowledgements
We thank J. Yuan for critical discussions and insightful comments on the manuscript. We thank staff members of Shanghai Synchrotron Radiation Facility BL17U for assistance in X-ray data collection. We thank the Tsinghua University Branch of China National Center for Protein Sciences Beijing for providing facility support in cryo-EM. This work was supported by the State High-Tech Development Plan (the “863 Program”) (2015AA020907 to C.L.), the National Natural Science Foundation (NSF) of China (31470748 to C.L. and 31570730 to X. Li), the Major State Basic Research Development Program (2016YFA0501902 to C.L. and X. Li, and 2016YFA0501902 to X. Li), the Chinese Academy of Sciences (to C.L.), The “1000 Talents Plan” of China to C.L. and X. Li, the Advanced Innovation Center for Structural Biology (to X. Li), and the Tsinghua-Peking Joint Center for Life Sciences (to X. Li).
Author information
Authors and Affiliations
Contributions
C.L., X. Li, D.L., and F.L. designed the project. X.G., F.L., J.G., and Y.L. performed crystallization, imaging, and biochemical assays. F.L., H.Z., X. Liu and M.Z. collected the data and determined the crystal structures. F.L., D.L., X. Li and C.L. analyzed the data and contributed to manuscript discussion and editing. D.L. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
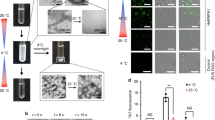
Supplementary Figure 1 Reversible liquid–liquid phase separation of WT FUS LC upon temperature change.
Protein concentration was 50 μM. Liquid droplets were visualized by DIC microscopy.
Supplementary Figure 2 Comparison of X-ray and electron diffraction of FUS RAC1 crystals.
Due to the clustering of the nano-sized crystals of RAC1, single crystal diffraction was very difficult to achieve by X-ray diffraction. Aided by electron microscope, we can possibly choose single crystal on the sample grid for diffraction. And ultrahigh resolution diffractions of RAC1 crystals were obtained.
Supplementary Figure 3 Structure families of amyloid fibrils.
The amyloid fibril spine of Tau VQIVYK represents a typical steric-zipper architecture shared by pathogenic amyloid fibrils (highlighted in green). PSMα3 has been recently found to form amyloid fibrils with α-helices rather than β-strands (highlighted in blue). The amyloid fibril spines of RAC1 showed a distinctive structure with no regular secondary structures (highlighted in orange). The amyloid fibril spines of RAC2 harbors water molecules between the interface of mating sheets rather than typical dry interface as seen in steric zippers (highlighted in yellow). PDB ID of VQIVYK is 2ON9. PDB ID of PSMα3 is 5I55.
Supplementary Figure 4 Structure of one layer of the FUS RAC1 fibril spine.
The hydroxyl group of Tyr38 forms hydrogen bonds with the carbonyl group of Tyr41 from the same segment and the hydroxyl group of Ser42 from the beneath segment. The segment is organized repetitively along the fibril axis. Five segment layers are depicted in different colors. Theoretically, fibrils can contain tens of thousands of layers.
Supplementary Figure 5 Powder X-ray diffraction images of RAC2 WT and Y58F fibrils and pathogenic fibrils of Tau VQIVYK.
FUS RAC2 fibrils contain a remarkably large distance between mating sheet of fibril spine. Y58F mutant of RAC2 forms fibrils with an apparently different diffraction pattern from the wild-type, indicating different fibril morphologies. The crystal structure of VQIVYK is from PDB ID: 2ON9. The inter-sheet distance of the structure model is measured between close Cα atoms of the mating sheets.
Supplementary Figure 6 Thermostability of amyloid fibrils formed by key segments of different amyloid proteins.
(a) FUS RAC1 and RAC2 segments form temperature-reversible amyloid fibrils. Mutation of RAC2 Y58F caused the formation of irreversible fibrils. Mutation of RAC2 Y58A prohibited the fibril assembly of RAC2. Some fibrils (thinner fibrils) formed by prion segment NNQQNY also dissolved as temperature increased. In contrast, pathogenic segments of Tau and Aβ formed highly thermal stable fibrils that are deemed to be closely related to AD. The scale bars are 200 nm. (b) Quantification of fibrils as temperature increases. The amount of fibrils was quantified by measuring the soluble peptide concentrations in the supernatant after ultracentrifugation (50,000 rpm for 50 min. Fibrils formed at 4 °C were ultracentrifuged at 4 °C; fibrils form at 20 °C and 50 °C were ultracentrifuged at 20 °C) at 205 nm using NANODROP 2000C (Thermo Scientific). Date shown are means and s.d. of n = 3 biologically independent samples.
Supplementary Figure 7 MALDI MS of FUS LC. The mass of purified recombinant LC is in red.
After incubation with DNA-PK (20 ng per μg LC) at 30 °C for 1 hour, the mass of LC increases indicating phosphorylation of LC (magenta). Subsequently, the phosphorylated LC was incubated with CIAP (10 U per 30 μg phosphorylated LC) at 30 °C for 30 min. The recovery of LC mass indicates dephosphorylation (blue).
Supplementary Figure 8 Structure models of phosphorylated FUS RAC1 and RAC2.
Phosphorylation of Ser42 would break the hydrogen bonds between Ser42 and Tyr38 that stabilize the rolled-sheet conformation of RAC1, and phosphorylation also hinders the interdigitation of mating sheets due to steric effects. In comparison, phosphorylation of Ser39 that is sticking outward of the fibril spine would not interfere the fibril structure.
Supplementary Figure 9 RACs in the structure of the FUS LC fibril core.
(a) Superposition of the 20 low-energy solid state NMR structural models of one FUS (37–97) molecule (PDB ID:5W3N). The fibril axis was indicated. The main chain conformation of FUS (37–97) is shown. RAC1 and RAC2 are highlighted in red. RAC1 and RAC2 are the most flexible regions in the structure. Segments 84SQSSQS89 and 90SYGQQS95 that are involved in the stable core of FUS (37–97) are highlighted in blue and cyan, respectively. (b) Segments 84SQSSQS89 and 90SYGQQS95 form thermostable crystal-like fibril bundles imaged by TEM. (c) Clouding points of LC variants (deletions of 84SQSSQS89 and 90SYGQQS95, respectively) from phase separation diagrams measured at the protein concentration of 150 μM. Data shown are means and s.d. of n = 3 biologically independent samples. Values were compared using Student’s t-test. “NS” represents “non-significant”.
Supplementary Figure 10 The interspine interaction in the crystal structure of RAC1 and RAC2.
The crystal lattices of RAC1 (a) and RAC2 (b) are viewed down the fibril spine. One unit of fibril spine is shown in black. Tyr pointing inward the fibril spine is shown in blue. Tyr sticking outward is shown in magenta. The zoom-in views show the detailed interactions between two adjacent spines (in the yellow frames). The inter-spine π-π interaction may reflect hybrid interactions of RAC1 and RAC2 with other segments of FUS.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 and Supplementary Tables 1 and 2
Rights and permissions
About this article
Cite this article
Luo, F., Gui, X., Zhou, H. et al. Atomic structures of FUS LC domain segments reveal bases for reversible amyloid fibril formation. Nat Struct Mol Biol 25, 341–346 (2018). https://doi.org/10.1038/s41594-018-0050-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-018-0050-8
This article is cited by
-
Alternative low-populated conformations prompt phase transitions in polyalanine repeat expansions
Nature Communications (2024)
-
A chaperone-like function of FUS ensures TAZ condensate dynamics and transcriptional activation
Nature Cell Biology (2024)
-
A solid beta-sheet structure is formed at the surface of FUS droplets during aging
Nature Chemical Biology (2024)
-
Synthesis and functionalization of scalable and versatile 2D protein films via amyloid-like aggregation
Nature Protocols (2024)
-
Benchmarking of force fields to characterize the intrinsically disordered R2-FUS-LC region
Scientific Reports (2023)