Abstract
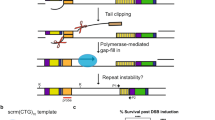
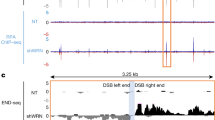
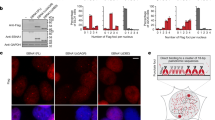
We developed an experimental system for studying genome instability caused by fragile X (CGG)n repeats in mammalian cells. Our method uses a selectable cassette carrying the HyTK gene under the control of the FMR1 promoter with (CGG)n repeats in its 5′ UTR, which is integrated into the unique RL5 site in murine erythroid leukemia cells. Carrier-size (CGG)n repeats markedly elevated the frequency of reporter inactivation, making cells ganciclovir resistant. These resistant clones had a unique mutational signature: a change in repeat length concurrent with mutagenesis in the reporter gene. Inactivation of genes implicated in break-induced replication, including Pold3, Pold4, Rad52, Rad51, and Smarcal1, reduced the frequency of ganciclovir-resistant clones to the baseline level that was observed in the absence of (CGG)n repeats. We propose that replication fork collapse at carrier-size (CGG)n repeats can trigger break-induced replication, which results in simultaneous repeat length changes and mutagenesis at a distance.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Carvalho, C. M. & Lupski, J. R. Mechanisms underlying structural variant formation in genomic disorders. Nat. Rev. Genet. 17, 224–238 (2016).
Mirkin, S. M. Expandable DNA repeats and human disease. Nature 447, 932–940 (2007).
Ashley, C. T. Jr & Warren, S. T. Trinucleotide repeat expansion and human disease. Annu. Rev. Genet. 29, 703–728 (1995).
Bidichandani, S. I. et al. Somatic sequence variation at the Friedreich ataxia locus includes complete contraction of the expanded GAA triplet repeat, significant length variation in serially passaged lymphoblasts and enhanced mutagenesis in the flanking sequence. Hum. Mol. Genet. 8, 2425–2436 (1999).
Chong, S. S. et al. Gametic and somatic tissue-specific heterogeneity of the expanded SCA1 CAG repeat in spinocerebellar ataxia type 1. Nat. Genet. 10, 344–350 (1995).
Monckton, D. G., Wong, L. J., Ashizawa, T. & Caskey, C. T. Somatic mosaicism, germline expansions, germline reversions and intergenerational reductions in myotonic dystrophy males: small pool PCR analyses. Hum. Mol. Genet. 4, 1–8 (1995).
McMurray, C. T. Mechanisms of trinucleotide repeat instability during human development. Nat. Rev. Genet. 11, 786–799 (2010).
Usdin, K., House, N. C. M. & Freudenreich, C. H. Repeat instability during DNA repair: insights from model systems. Crit. Rev. Biochem. Mol. Biol. 50, 142–167 (2015).
Kim, J. C. & Mirkin, S. M. The balancing act of DNA repeat expansions. Curr. Opin. Genet. Dev. 23, 280–288 (2013).
Freudenreich, C. H., Kantrow, S. M. & Zakian, V. A. Expansion and length-dependent fragility of CTG repeats in yeast. Science 279, 853–856 (1998).
Shah, K. A. & Mirkin, S. M. The hidden side of unstable DNA repeats: mutagenesis at a distance. DNA Repair 32, 106–112 (2015).
McGinty, R. J. et al. Nanopore sequencing of complex genomic rearrangements in yeast reveals mechanisms of repeat-mediated double-strand break repair. Genome Res. 27, 2072–2082 (2017).
Chandok, G. S., Patel, M. P., Mirkin, S. M. & Krasilnikova, M. M. Effects of Friedreich’s ataxia GAA repeats on DNA replication in mammalian cells. Nucleic Acids Res. 40, 3964–3974 (2012).
Follonier, C., Oehler, J., Herrador, R. & Lopes, M. Friedreich’s ataxia–associated GAA repeats induce replication-fork reversal and unusual molecular junctions. Nat. Struct. Mol. Biol. 20, 486–494 (2013).
Gerhardt, J. et al. Stalled DNA replication forks at the endogenous GAA repeats drive repeat expansion in Friedreich’s ataxia cells. Cell Rep. 16, 1218–1227 (2016).
Gerhardt, J. et al. The DNA replication program is altered at the FMR1 locus in fragile X embryonic stem cells. Mol. Cell 53, 19–31 (2014).
Liu, G., Chen, X., Bissler, J. J., Sinden, R. R. & Leffak, M. Replication-dependent instability at (CTG)·(CAG) repeat hairpins in human cells. Nat. Chem. Biol. 6, 652–659 (2010).
Liu, G. et al. Altered replication in human cells promotes DMPK (CTG)n · (CAG)n repeat instability. Mol. Cell. Biol. 32, 1618–1632 (2012).
Rindler, P. M., Clark, R. M., Pollard, L. M., De Biase, I. & Bidichandani, S. I. Replication in mammalian cells recapitulates the locus-specific differences in somatic instability of genomic GAA triplet-repeats. Nucleic Acids Res. 34, 6352–6361 (2006).
Seriola, A. et al. Huntington’s and myotonic dystrophy hESCs: down-regulated trinucleotide repeat instability and mismatch repair machinery expression upon differentiation. Hum. Mol. Genet. 20, 176–185 (2011).
Ku, S. et al. Friedreich’s ataxia induced pluripotent stem cells model intergenerational GAA·TTC triplet repeat instability. Cell Stem Cell 7, 631–637 (2010).
Hansen, R. S., Canfield, T. K., Lamb, M. M., Gartler, S. M. & Laird, C. D. Association of fragile X syndrome with delayed replication of the FMR1 gene. Cell 73, 1403–1409 (1993).
Manley, K., Shirley, T. L., Flaherty, L. & Messer, A. Msh2 deficiency prevents in vivo somatic instability of the CAG repeat in Huntington disease transgenic mice. Nat. Genet. 23, 471–473 (1999).
Kovtun, I. V. & McMurray, C. T. Trinucleotide expansion in haploid germ cells by gap repair. Nat. Genet. 27, 407–411 (2001).
Savouret, C. et al. CTG repeat instability and size variation timing in DNA repair–deficient mice. EMBO J. 22, 2264–2273 (2003).
Ezzatizadeh, V. et al. The mismatch repair system protects against intergenerational GAA repeat instability in a Friedreich ataxia mouse model. Neurobiol. Dis. 46, 165–171 (2012).
Zhao, X.-N. et al. Mutsβ generates both expansions and contractions in a mouse model of the fragile X–associated disorders. Hum. Mol. Genet. 24, 7087–7096 (2015).
Wöhrle, D. et al. Heterogeneity of DM kinase repeat expansion in different fetal tissues and further expansion during cell proliferation in vitro: evidence for a casual involvement of methyl-directed DNA mismatch repair in triplet repeat stability. Hum. Mol. Genet. 4, 1147–1153 (1995).
Du, J. et al. Role of mismatch repair enzymes in GAA·TTC triplet-repeat expansion in Friedreich ataxia induced pluripotent stem cells. J. Biol. Chem. 287, 29861–29872 (2012).
Halabi, A., Ditch, S., Wang, J. & Grabczyk, E. DNA mismatch repair complex MutSβ promotes GAA·TTC repeat expansion in human cells. J. Biol. Chem. 287, 29958–29967 (2012).
Lin, Y., Dent, S. Y., Wilson, J. H., Wells, R. D. & Napierala, M. R loops stimulate genetic instability of CTG·CAG repeats. Proc. Natl. Acad. Sci. USA 107, 692–697 (2010).
Nakamori, M., Pearson, C. E. & Thornton, C. A. Bidirectional transcription stimulates expansion and contraction of expanded (CTG)·(CAG) repeats. Hum. Mol. Genet. 20, 580–588 (2011).
Zhao, X.-N. & Usdin, K. Gender and cell-type-specific effects of the transcription-coupled repair protein, ERCC6/CSB, on repeat expansion in a mouse model of the fragile X–related disorders. Hum. Mutat. 35, 341–349 (2014).
Chan, N. L. et al. The Werner syndrome protein promotes CAG/CTG repeat stability by resolving large (CAG)n/(CTG)n hairpins. J. Biol. Chem. 287, 30151–30156 (2012).
Frizzell, A. et al. RTEL1 inhibits trinucleotide repeat expansions and fragility. Cell Rep. 6, 827–835 (2014).
Gorbunova, V. et al. Selectable system for monitoring the instability of CTG/CAG triplet repeats in mammalian cells. Mol. Cell. Biol. 23, 4485–4493 (2003).
Lin, Y., Dion, V. & Wilson, J. H. Transcription promotes contraction of CAG repeat tracts in human cells. Nat. Struct. Mol. Biol. 13, 179–180 (2006).
Lin, Y. & Wilson, J. H. Transcription-induced CAG repeat contraction in human cells is mediated in part by transcription-coupled nucleotide excision repair. Mol. Cell. Biol. 27, 6209–6217 (2007).
Chatterjee, N., Lin, Y., Santillan, B. A., Yotnda, P. & Wilson, J. H. Environmental stress induces trinucleotide repeat mutagenesis in human cells. Proc. Natl. Acad. Sci. USA 112, 3764–3769 (2015).
Fu, Y. H. et al. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell 67, 1047–1058 (1991).
Feng, Y. Q., Lorincz, M. C., Fiering, S., Greally, J. M. & Bouhassira, E. E. Position effects are influenced by the orientation of a transgene with respect to flanking chromatin. Mol. Cell. Biol. 21, 298–309 (2001).
Costantino, L. et al. Break-induced replication repair of damaged forks induces genomic duplications in human cells. Science 343, 88–91 (2014).
Voineagu, I., Surka, C. F., Shishkin, A. A., Krasilnikova, M. M. & Mirkin, S. M. Replisome stalling and stabilization at CGG repeats, which are responsible for chromosomal fragility. Nat. Struct. Mol. Biol. 16, 226–228 (2009).
Ebersole, T. et al. tRNA genes protect a reporter gene from epigenetic silencing in mouse cells. Cell Cycle 10, 2779–2791 (2011).
Kim, J. C., Harris, S. T., Dinter, T., Shah, K. A. & Mirkin, S. M. The role of break-induced replication in large-scale expansions of (CAG)n/(CTG)n repeats. Nat. Struct. Mol. Biol. 24, 55–60 (2017).
Sakofsky, C. J. et al. Break-induced replication is a source of mutation clusters underlying kataegis. Cell Rep. 7, 1640–1648 (2014).
Sotiriou, S. K. et al. Mammalian RAD52 functions in break-induced replication repair of collapsed DNA replication forks. Mol. Cell 64, 1127–1134 (2016).
Lugli, N., Sotiriou, S. K. & Halazonetis, T. D. The role of SMARCAL1 in replication fork stability and telomere maintenance. DNA Repair 56, 129–134 (2017).
Wang, G. & Vasquez, K. M. Impact of alternative DNA structures on DNA damage, DNA repair, and genetic instability. DNA Repair 19, 143–151 (2014).
Malkova, A. & Ira, G. Break-induced replication: functions and molecular mechanism. Curr. Opin. Genet. Dev. 23, 271–279 (2013).
Roumelioti, F. M. et al. Alternative lengthening of human telomeres is a conservative DNA replication process with features of break-induced replication. EMBO Rep. 17, 1731–1737 (2016).
Pasero, P. & Vindigni, A. Nucleases acting at stalled forks: how to reboot the replication program with a few shortcuts. Annu. Rev. Genet. 51, 477–499 (2017).
Smith, C. E., Llorente, B. & Symington, L. S. Template switching during break-induced replication. Nature 447, 102–105 (2007).
de Graaff, E. et al. Hotspot for deletions in the CGG repeat region of FMR1 in fragile X patients. Hum. Mol. Genet. 4, 45–49 (1995).
Nichol Edamura, K. & Pearson, C. E. DNA methylation and replication: implications for the “deletion hotspot” region of FMR1. Hum. Genet. 118, 301–304 (2005).
Hagerman, R. J. & Hagerman, P. J. The fragile X premutation: into the phenotypic fold. Curr. Opin. Genet. Dev. 12, 278–283 (2002).
Sherman, S. L. Premature ovarian failure among fragile X premutation carriers: parent-of-origin effect? Am. J. Hum. Genet. 67, 11–13 (2000).
Man, L., Lekovich, J., Rosenwaks, Z. & Gerhardt, J. Fragile X–associated diminished ovarian reserve and primary ovarian insufficiency from molecular mechanisms to clinical manifestations. Front. Mol. Neurosci. 10, 290 (2017).
Hagerman, R., Au, J. & Hagerman, P. FMR1 premutation and full mutation molecular mechanisms related to autism. J. Neurodev. Disord. 3, 211–224 (2011).
Kumari, D. & Usdin, K. Interaction of the transcription factors USF1, USF2, and alpha-Pal/Nrf-1 with the FMR1 promoter. Implications for fragile X mental retardation syndrome. J. Biol. Chem. 276, 4357–4364 (2001).
Kononenko, A. V. et al. A portable BRCA1-HAC (human artificial chromosome) module for analysis of BRCA1 tumor suppressor function. Nucleic Acids Res. 42, e164 (2014).
Kim, J.-H. et al. Human gamma-satellite DNA maintains open chromatin structure and protects a transgene from epigenetic silencing. Genome Res. 19, 533–544 (2009).
Acknowledgements
We thank K. Usdin (NIDDK, NIH) for the generous gift of the p32.9 plasmid, E. Bouhassira (Albert Einstein College of Medicine) for providing us with the RL5 cell line, D. Gennert for technical assistance, and A. Neil for his invaluable editorial help. This work was supported by NIH grants R01GM60987 and P01GM105473 to S.M.M. and R01CA093729 to K.M.V.
Author information
Authors and Affiliations
Contributions
A.V.K., T.E., K.M.V. and S.M.M. designed the study; A.V.K. and T.E. performed experiments; A.V.K., T.E. and S.M.M. analyzed data; A.V.K., T.E., K.M.V. and S.M.M. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 Mutations found in the TK domain of the HyTK gene in various FMR1-(CGG)n-HyTK cassettes.
DNA bases in the TK domain of the wild-type HyTK gene that underwent mutagenesis are shown in bold. Mutations found in the cassettes with (CGG)0, (CGG)53, and (CGG)153 repeats are shown in green, blue, and red, respectively; mutations found in the cassette with (CGG)153 repeats upon treatment with Pold3 siRNA are shown in orange; and G7 and C6 mutation hotspots are highlighted in yellow. V, insertion; Δ, deletion; D, duplication; FV, insertion to frameshift; FΔ, deletion to frameshift; X, complex mutation; AT and TT, tandem base substitutions.
Supplementary Figure 2 PCR analysis of repeat lengths for clones originated after treatment of the FMR1-(CGG)153-HyTK cell line with Pold3 siRNA.
Lane 1 shows the original repeat; lanes 2–4, 6, 8, and 9 show expanded repeats; and lanes 5 and 7 show unchanged repeat. Mutations found within the HyTK gene are shown at the top of the corresponsing lane.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1 and 2, and Supplementary Table 1
Rights and permissions
About this article
Cite this article
Kononenko, A.V., Ebersole, T., Vasquez, K.M. et al. Mechanisms of genetic instability caused by (CGG)n repeats in an experimental mammalian system. Nat Struct Mol Biol 25, 669–676 (2018). https://doi.org/10.1038/s41594-018-0094-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-018-0094-9
This article is cited by
-
DNA polymerase stalling at structured DNA constrains the expansion of short tandem repeats
Genome Biology (2020)
-
Break-induced replication sparks CGG-repeat instability
Nature Structural & Molecular Biology (2018)