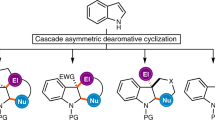
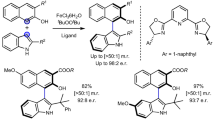
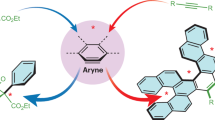
Abstract
Atropisomeric architectures are increasingly encountered in modern materials and medicinally important compounds. More importantly, they are now a characteristic of broadly useful chiral ligands and organocatalysts. Over the past decade, substantial advancements have been made in enhancing the accessibility of major classes of atropisomers through the refinement of existing strategies and the introduction of contemporary concepts for catalytic atroposelective synthesis. This synthetic capability enables the expansion of chemical space and facilitates the preparation of valuable atropisomeric scaffolds. Here we review the state of the art in the asymmetric synthesis of atropisomers with the help of selected examples. Focus will be placed on the strategies that have emerged rapidly in recent years, and that are characterized by high versatility and modularity. Additionally, the incorporation of emerging synthetic tools and representative scaffolds are discussed, alongside future directions in this research domain.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Christie, G. H. & Kenner, J. LXXI.—The molecular configurations of polynuclear aromatic compounds. Part I. The resolution of γ-6:6′-dinitro- and 4:6:4′:6′-tetranitrodiphenic acids into optically active components. J. Chem. Soc. Trans. 120, 614–620 (1922).
LaPlante, S. R., Edwards, P. J., Fader, L. D., Jakalian, A. & Hucke, O. Revealing atropisomer axial chirality in drug discovery. ChemMedChem 6, 505–513 (2011).
Noyori, R. & Takaya, H. BINAP: an efficient chiral element for asymmetric catalysis. Acc. Chem. Res. 23, 345–350 (1990).
Chen, Y., Yekta, S. & Yudin, A. K. Modified BINOL ligands in asymmetric catalysis. Chem. Rev. 103, 3155–3212 (2003).
Teichert, J. F. & Feringa, B. L. Phosphoramidites: privileged ligands in asymmetric catalysis. Angew. Chem. Int. Ed. 49, 2486–2528 (2010).
Rokade, B. V. & Guiry, P. J. Axially chiral P,N-ligands: some recent twists and turns. ACS Catal. 8, 624–643 (2018).
Shirakawa, S. & Maruoka, K. Recent developments in asymmetric phase-transfer reactions. Angew. Chem. Int. Ed. 52, 4312–4348 (2013).
Akiyama, T. Stronger Brønsted acids. Chem. Rev. 107, 5744–5758 (2007).
Parmar, D., Sugiono, E., Raja, S. & Rueping, M. Complete field guide to asymmetric BINOL-phosphate derived Brønsted acid and metal catalysis: history and classification by mode of activation; Brønsted acidity, hydrogen bonding, ion pairing and metal phosphates. Chem. Rev. 114, 9047–9153 (2014).
Kumarasamy, E., Raghunathan, R., Sibi, M. K. & Sivaguru, J. Nonbiaryl and heterobiaryl atropisomers: molecular templates with promise for atropselective chemical transformations. Chem. Rev. 115, 11239–11300 (2015).
Wencel-Delord, J., Panossian, A., Leroux, F. R. & Colobert, F. Recent advances and new concepts for the synthesis of axially stereoenriched biaryls. Chem. Soc. Rev. 44, 3418–3430 (2015).
Cheng, J. K., Xiang, S.-H., Li, S., Ye, L. & Tan, B. Recent advances in catalytic asymmetric construction of atropisomers. Chem. Rev. 121, 4805–4902 (2021).
Lassaletta, J. M. Atropisomerism and Axial Chirality (World Scientific, 2019).
Tan, B. Axially Chiral Compounds: Asymmetric Synthesis and Applications (Wiley, 2021).
Zheng, J. & You, S.-L. Construction of axial chirality by rhodium-catalyzed asymmetric dehydrogenative heck coupling of biaryl compounds with alkenes. Angew. Chem. Int. Ed. 53, 13244–13247 (2014).
Liu, C.-X., Zhang, W.-W., Yin, S.-Y., Gu, Q. & You, S.-L. Synthesis of atropisomers by transition-metal-catalyzed asymmetric C-H functionalization reactions. J. Am. Chem. Soc. 143, 14025–14040 (2021).
Luo, J. et al. Enantioselective synthesis of biaryl atropisomers by Pd-catalyzed C-H olefination using chiral spiro phosphoric acid ligands. Angew. Chem. Int. Ed. 58, 6708–6712 (2019).
Tian, M., Bai, D., Zheng, G., Chang, J. & Li, X. Rh(III)-catalyzed asymmetric synthesis of axially chiral biindolyls by merging C-H activation and nucleophilic cyclization. J. Am. Chem. Soc. 141, 9527–9532 (2019).
Yao, Q.-J., Zhang, S., Zhan, B.-B. & Shi, B.-F. Atroposelective synthesis of axially chiral biaryls by palladium-catalyzed asymmetric C-H olefination enabled by a transient chiral auxiliary. Angew. Chem. Int. Ed. 56, 6617–6621 (2017).
Liao, G., Zhou, T., Yao, Q.-J. & Shi, B.-F. Recent advances in the synthesis of axially chiral biaryls via transition metal-catalysed asymmetric C-H functionalization. Chem. Commun. 55, 8514–8523 (2019).
Dhawa, U. et al. Enantioselective pallada-electrocatalyzed C-H activation by transient directing groups: expedient access to helicenes. Angew. Chem. Int. Ed. 59, 13451–13457 (2020). The successful merging of electro-chemical synthesis and asymmetric catalysis in atroposelective synthesis.
Dhawa, U. et al. Enantioselective palladaelectro-catalyzed C-H olefinations and allylations for N-C axial chirality. Chem. Sci. 12, 14182–14188 (2021).
Jang, Y.-S., Woźniak, Ł., Pedroni, J. & Cramer, N. Access to P- and axially chiral biaryl phosphine oxides by enantioselective CpxIrIII-catalyzed C-H arylations. Angew. Chem. Int. Ed. 57, 12901–12905 (2018).
Serrano, J., Ros, A. & Lassaletta, J. M. Ir-catalyzed atroposelective desymmetrization of heterobiaryls: hydroarylation of vinyl ethers and bicycloalkenes. J. Am. Chem. Soc. 142, 2628–2639 (2020).
Uchikura, T. et al. Chiral phosphoric acid-palladium(II) complex catalyzed asymmetric desymmetrization of biaryl compounds by C(sp3)-H activation. J. Am. Chem. Soc. 145, 15906–15911 (2023).
Jacob, N., Zaid, Y., Oliveira, J. C. A., Ackermann, L. & Wencel-Delord, J. Cobalt-catalyzed enantioselective C-H arylation of indoles. J. Am. Chem. Soc. 144, 798–806 (2022).
Choppin, S. & Wencel-Delord, J. Sulfoxide-directed or 3d-metal catalyzed C-H activation and hypervalent iodines as tools for atroposelective synthesis. Acc. Chem. Res. 56, 189–202 (2023).
Wu, Y.-J. et al. Synthesis of axially chiral biaryls through cobalt(II)-catalyzed atroposelective C-H arylation. Angew. Chem. Int. Ed. 62, e202310004 (2023).
Catellani, M., Frignani, F. & Rangoni, A. A complex catalytic cycle leading to a regioselective synthesis of o,o′-disubstituted vinylarenes. Angew. Chem. Int. Ed. 36, 119–122 (1997).
Ding, L., Sui, X. & Gu, Z. Enantioselective synthesis of biaryl atropisomers via Pd/norbornene-catalyzed three-component cross-couplings. ACS Catal. 8, 5630–5635 (2018).
Liu, Z.-S. et al. Construction of axial chirality via palladium/chiral norbornene cooperative catalysis. Nat. Catal. 3, 727–733 (2020). The generation of atropisomeric Pd(II)–biaryl complex for further transformation.
Feng, Q. et al. Catalytic atroposelective Catellani reaction enables construction of axially chiral biaryl monophosphine oxides. CCS Chem. 3, 377–387 (2021).
Liu, Z.-S. et al. An axial-to-axial chirality transfer strategy for atroposelective construction of C-N axial chirality. Chem 7, 1917–1932 (2021).
Gao, Q. et al. Catalytic synthesis of atropisomeric o-terphenyls with 1,2-diaxes via axial-to-axial diastereoinduction. J. Am. Chem. Soc. 143, 7253–7260 (2021).
Bringmann, G. & Hartung, T. First atropo‐enantioselective ring opening of achiral biaryls containing lactone bridges with chiral hydride‐transfer reagents derived from borane. Angew. Chem. Int. Ed. 31, 761–762 (1992).
Ashizawa, T., Tanaka, S. & Yamada, T. Catalytic atropo-enantioselective reduction of biaryl lactones to axially chiral biaryl compounds. Org. Lett. 10, 2521–2524 (2008).
Chen, G.-Q. et al. Design and synthesis of chiral oxa-spirocyclic ligands for Ir-catalyzed direct asymmetric reduction of Bringmann’s lactones with molecular H2. J. Am. Chem. Soc. 140, 8064–8068 (2018).
Yu, C., Huang, H., Li, X., Zhang, Y. & Wang, W. Dynamic kinetic resolution of biaryl lactones via a chiral bifunctional amine thiourea-catalyzed highly atropoenantioselective transesterification. J. Am. Chem. Soc. 138, 6956–6959 (2016).
Mori, K., Itakura, T. & Akiyama, T. Enantiodivergent atroposelective synthesis of chiral biaryls by asymmetric transfer hydrogenation: chiral phosphoric acid catalyzed dynamic kinetic resolution. Angew. Chem. Int. Ed. 55, 11642–11646 (2016).
Zhang, J. & Wang, J. Atropoenantioselective redox-neutral amination of biaryl compounds through borrowing hydrogen and dynamic kinetic resolution. Angew. Chem. Int. Ed. 57, 465–469 (2018).
Zhao, K. et al. Enhanced reactivity by torsional strain of cyclic diaryliodonium in Cu-catalyzed enantioselective ring-opening reaction. Chem 4, 599–612 (2018). The introduction of cyclic diaryliodonium for catalytic asymmetric synthesis of biaryls.
Xue, X. & Gu, Z. Synthesis of bridged biaryl atropisomers via sequential Cu- and Pd-catalyzed asymmetric ring opening and cyclization. Org. Lett. 21, 3942–3945 (2019).
Hou, M., Deng, R. & Gu, Z. Cu-catalyzed enantioselective atropisomer synthesis via thiolative ring opening of five-membered cyclic diaryliodoniums. Org. Lett. 20, 5779–5783 (2018).
Duan, L., Zhao, K., Wang, Z., Zhang, F.-L. & Gu, Z. Enantioselective ring-opening/oxidative phosphorylation and P‑transfer reaction of cyclic diaryliodoniums. ACS Catal. 9, 9852–9858 (2019).
Duan, L., Wang, Z., Zhao, K. & Gu, Z. Enantioselective preparation of atropisomeric biaryl trifluoromethylsulfanes via ring-opening of cyclic diaryliodoniums. Chem. Commun. 57, 3881–3884 (2021).
Zhao, K., Yang, S., Gong, Q., Duan, L. & Gu, Z. Diols activation via Cu/borinic acids synergistic catalysis in atroposelective ring-opening of cyclic diaryliodoniums. Angew. Chem. Int. Ed. 60, 5788–5793 (2021).
Wu, M. et al. Organocatalytic Si-CAryl bond functionalization-enabled atroposelective synthesis of axially chiral biaryl siloxanes. J. Am. Chem. Soc. 145, 20646–20654 (2023).
Li, C., Chen, D. & Tang, W. Addressing the challenges in Suzuki-Miyaura cross-couplings by ligand design. Synlett 27, 2183–2200 (2016).
Goetzke, F. W., Van Dijk, L. & Fletcher, S. P. in Patai’s Chemistry of Functional Groups (Rappoport, Z. ed.) 1−54 (Wiley, 2019).
Hedouin, G., Hazra, S., Gallou, F. & Handa, S. The catalytic formation of atropisomers and stereocenters via asymmetric Suzuki-Miyaura couplings. ACS Catal. 12, 4918–4937 (2022).
Cherney, A. H., Kadunce, N. T. & Reisman, S. E. Enantioselective and enantiospecific transition-metal-catalyzed cross-coupling reactions of organometallic reagents to construct C-C bonds. Chem. Rev. 115, 9587–9652 (2015).
Loxq, P., Manoury, E., Poli, R., Deydier, E. & Labande, A. Synthesis of axially chiral biaryl compounds by asymmetric catalytic reactions with transition metals. Coord. Chem. Rev. 308, 131–190 (2016).
Surgenor, R. R., Liu, X., Keenlyside, M. J. H., Myers, W. & Smith, M. D. Enantioselective synthesis of atropisomeric indoles via iron-catalysed oxidative cross-coupling. Nat. Chem. 15, 357–365 (2023).
Zhang, X., Wang, J. & Yang, S.-D. Enantioselective cobalt-catalyzed reductive cross-coupling for the synthesis of axially chiral phosphine-olefin ligands. ACS Catal. 11, 14008–14015 (2021).
Akiyama, T., Itoh, J., Yokota, K. & Fuchibe, K. Enantioselective Mannich-type reaction catalyzed by a chiral Brønsted acid. Angew. Chem. Int. Ed. 43, 1566–1568 (2004).
Uraguchi, D. & Terada, M. Chiral Brønsted acid-catalyzed direct Mannich reactions via electrophilic activation. J. Am. Chem. Soc. 126, 5356–5357 (2004).
Qi, L.-W., Mao, J.-H., Zhang, J. & Tan, B. Organocatalytic asymmetric arylation of indoles enabled by azo groups. Nat. Chem. 10, 58–64 (2018). The identification of azo group for the arene umpolung under CPA catalysis and its application in the synthesis of atropisomers.
Qi, L.-W., Li, S., Xiang, S.-H., Wang, J. & Tan, B. Asymmetric construction of atropisomeric binaphthyls via a redox neutral cross-coupling strategy. Nat. Catal. 2, 314–323 (2019).
Xia, W. et al. Chiral phosphoric acid catalyzed atroposelective C-H amination of arenes. Angew. Chem. Int. Ed. 59, 6775–6779 (2020).
Mao, J.-H. et al. Organocatalyst-controlled site-selective arene C-H functionalization. Nat. Chem. 13, 982–991 (2021).
Da, B.-C., Wang, Y.-B., Cheng, C. K., Xiang, S.-H. & Tan, B. Organocatalytic atroposelective cross-coupling of 1-azonaphthalenes and 2-naphthols. Angew. Chem. Int. Ed. 62, e202303128 (2023).
Ding, W.-Y. et al. DFT-guided phosphoric-acid-catalyzed atroposelective arene functionalization of nitrosonaphthalene. Chem 6, 2046–2059 (2020).
Zhang, H.-H. et al. Design and enantioselective construction of axially chiral naphthyl-indole skeletons. Angew. Chem. Int. Ed. 56, 116–121 (2017).
Wang, J.-Y. et al. Atroposelective construction of axially chiral alkene‐indole scaffolds via catalytic enantioselective addition reaction of 3‐alkynyl‐2‐indolylmethanols. Chin. J. Chem. 39, 2163–2171 (2021).
Rodriguez, J. & Bonne, D. Enantioselective organocatalytic activation of vinylidene-quinone methides (VQMs). Chem. Commun. 55, 11168–11170 (2019).
Furusawa, M. et al. Base-catalyzed Schmittel cycloisomerization of o-phenylenediyne-linked bis(arenol)s to indeno[1,2-c]chromenes. Tetrahedron Lett. 54, 7107–7110 (2013).
Liu, Y. et al. Organocatalytic atroposelective intramolecular [4 + 2] cycloaddition: synthesis of axially chiral heterobiaryls. Angew. Chem. Int. Ed. 57, 6491–6495 (2018). The realization of enantiocontrolled nucleophilic addition to VQMs to synthesize atropisomers by judicious modification of the chiral catalyst.
Peng, L. et al. Organocatalytic asymmetric annulation of ortho-alkynylanilines: synthesis of axially chiral naphthyl-C2-indoles. Angew. Chem. Int. Ed. 58, 17199–17204 (2019).
Arae, S. et al. Asymmetric synthesis of axially chiral benzocarbazole derivatives based on catalytic enantioselective hydroarylation of alkynes. Org. Lett. 20, 4796–4800 (2018).
Zhang, L. et al. Design and atroposelective construction of IAN analogues via organocatalytic asymmetric heteroannulation of alkynes. Angew. Chem. Int. Ed. 59, 23077–23082 (2020).
Jia, S. et al. Organocatalytic enantioselective construction of axially chiral sulfone-containing styrenes. J. Am. Chem. Soc. 140, 7056–7060 (2018).
Wang, Y.-B. et al. Rational design, enantioselective synthesis and catalytic applications of axially chiral EBINOLs. Nat. Catal. 2, 504–513 (2019).
Xu, D. et al. Diversity-oriented enantioselective construction of atropisomeric heterobiaryls and N-aryl indoles via vinylidene ortho-quinone methides. CCS Chem. 4, 2686–2697 (2022).
Tan, Y. et al. Enantioselective construction of vicinal diaxial styrenes and multiaxis system via organocatalysis. J. Am. Chem. Soc. 140, 16893–16898 (2018).
Liu, H., Li, K., Huang, S. & Yan, H. An isolable vinylidene ortho-quinone methide: synthesis, structure and reactivity. Angew. Chem. Int. Ed. 61, e202117063 (2022).
Liang, Y. et al. Enantioselective construction of axially chiral amino sulfide vinyl arenes by chiral sulfide-catalyzed electrophilic carbothiolation of alkynes. Angew. Chem. Int. Ed. 59, 4959–4964 (2020).
Zhang, X. et al. Asymmetric azide-alkyne cycloaddition with Ir(I)/squaramide cooperative catalysis: atroposelective synthesis of axially chiral aryltriazoles. J. Am. Chem. Soc. 144, 6200–6207 (2022).
Guo, W.-T. et al. Enantioselective Rh-catalyzed azide-internal-alkyne cycloaddition for the construction of axially chiral 1,2,3-triazoles. J. Am. Chem. Soc. 144, 6981–6991 (2022).
Zeng, L., Li, J. & Cui, S. Rhodium-catalyzed atroposelective click cycloaddition of azides and alkynes. Angew. Chem. Int. Ed. 61, e202205037 (2022).
Irie, R., Masutani, K. & Katsuki, T. Asymmetric aerobic oxidative coupling of 2-naphthol derivatives catalyzed by photo-activated chiral (NO)Ru(II)-salen complex. Synlett 10, 1433–1436 (2000).
Hölzl-Hobmeier, A. et al. Catalytic deracemization of chiral allenes by sensitized excitation with visible light. Nature 564, 240–243 (2018).
Jiang, X. et al. Construction of axial chirality via asymmetric radical trapping by cobalt under visible light. Nat. Catal. 5, 788–797 (2022). The effective combination of photocatalysis with asymmetric catalysis in atroposelective synthesis.
Xiong, W. et al. Dynamic kinetic reductive conjugate addition for construction of axial chirality enabled by synergistic photoredox/cobalt catalysis. J. Am. Chem. Soc. 145, 7983–7991 (2023).
Jiang, H. et al. Photoinduced cobalt-catalyzed desymmetrization of dialdehydes to access axial chirality. J. Am. Chem. Soc. 145, 6944–6952 (2023).
Liang, D., Chen, J.-R., Tan, L.-P., He, Z.-W. & Xiao, W.-J. Catalytic asymmetric construction of axially and centrally chiral heterobiaryls by Minisci reaction. J. Am. Chem. Soc. 144, 6040–6049 (2022).
Meyer, T. H., Choi, I., Tian, C. & Ackermann, L. Powering the future: how can electrochemistry make a difference in organic synthesis? Chem 6, 2484–2496 (2020).
von Münchow, T., Dana, S., Xu, Y., Yuan, B. & Ackermann, L. Enantioselective electrochemical cobalt-catalyzed aryl C-H activation reactions. Science 379, 1036–1042 (2023).
Lin, Y., von Münchow, T. & Ackermann, L. Cobaltaelectro-catalyzed C-H annulation with allenes for atropochiral and P‑stereogenic compounds: late-stage diversification and continuous flow scale-up. ACS Catal. 13, 9713–9723 (2023).
Qiu, H. et al. Enantioselective Ni-catalyzed electrochemical synthesis of biaryl atropisomers. J. Am. Chem. Soc. 142, 9872–9878 (2020).
Bock, L. & Adams, R. The stereochemistry of N-phenyl pyrroles. The preparation and resolution of N-2-carboxyphenyl-2,5-dimethyl-3-carboxypyrrole. J. Am. Chem. Soc. 53, 374–376 (1931).
Frey, J., Choppin, S., Colobert, F. & Wencel-Delord, J. Towards atropoenantiopure N-C axially chiral compounds via stereoselective C-N bond formation. Chimia 74, 883–889 (2020).
Kitagawa, O. Chiral Pd-catalyzed enantioselective syntheses of various N-C axially chiral compounds and their synthetic applications. Acc. Chem. Res. 54, 719–730 (2021).
Rodríguez-Salamanca, P., Fernández, R., Hornillos, V. & Lassaletta, J. M. Asymmetric synthesis of axially chiral C-N atropisomers. Chem. Eur. J. 28, e202104442 (2022).
Zheng, S.-C. et al. Organocatalytic atroposelective synthesis of axially chiral styrenes. Nat. Commun. 8, 15238 (2017). Conformationally stable acyclic axially chiral alkenes were first accessed.
Feng, J., Li, B., He, Y. & Gu, Z. Enantioselective synthesis of atropisomeric vinyl arene compounds by palladium catalysis: a carbene strategy. Angew. Chem. Int. Ed. 55, 2186–2190 (2016).
Jolliffe, J. D., Armstrong, R. J. & Smith, M. D. Catalytic enantioselective synthesis of atropisomeric biaryls by a cation-directed O-alkylation. Nat. Chem. 9, 558–562 (2017).
Wu, S., Xiang, S.-H., Cheng, J. K. & Tan, B. Axially chiral alkenes: atroposelective synthesis and applications. Tetrahedron Chem. 1, 100009 (2022).
Yang, K. et al. Construction of axially chiral arylborons via atroposelective Miyaura borylation. J. Am. Chem. Soc. 143, 10048–10053 (2021). Conformationally stable atropisomers with a C-B stereogenic axis were accessed.
Yang, K. et al. Construction of C–B axial chirality via dynamic kinetic asymmetric cross-coupling mediated by tetracoordinate boron. Nat. Commun. 14, 4438 (2023).
Xu, J. et al. Palladium-catalyzed atroposelective kinetic C-H olefination and allylation for the synthesis of C-B axial chirality. Angew. Chem. Int. Ed. 62, e202313388 (2023).
Yang, J. et al. Chiral phosphoric acid-catalyzed remote control of axial chirality at boron-carbon bond. J. Am. Chem. Soc. 143, 12924–12929 (2021).
Mei, G.-J. et al. Rational design and atroposelective synthesis of N-N axially chiral compounds. Chem 7, 2743–2757 (2021).
Mei, G.-J., Koay, W. L., Guan, C.-Y. & Lu, Y. Atropisomers beyond the C-C axial chirality: advances in catalytic asymmetric synthesis. Chem 8, 1855–1893 (2022).
Centonze, G., Portolani, C., Righi, P. & Bencivenni, G. Enantioselective strategies for the synthesis of N-N atropisomers. Angew. Chem. Int. Ed. 62, e202303966 (2023).
Betson, M. S., Clayden, J., Worrall, C. P. & Peace, S. Three groups good, four groups bad? Atropisomerism in ortho-substituted diaryl ethers. Angew. Chem. Int. Ed. 45, 5803–5807 (2006).
Clayden, J., Senior, J. & Helliwell, M. Atropisomerism at C-S bonds: asymmetric synthesis of diaryl sulfones by dynamic resolution under thermodynamic control. Angew. Chem. Int. Ed. 48, 6270–6273 (2009).
Yuan, B. et al. Biocatalytic desymmetrization of an atropisomer with both an enantioselective oxidase and ketoreductases. Angew. Chem. Int. Ed. 49, 7010–7013 (2010).
Dai, L. et al. A dynamic kinetic resolution approach to axially chiral diaryl ethers by catalytic atroposelective transfer hydrogenation. Angew. Chem. Int. Ed. 62, e202216534 (2023).
Bao, H., Chen, Y. & Yang, X. Catalytic asymmetric synthesis of axially chiral diaryl ethers through enantioselective desymmetrization. Angew. Chem. Int. Ed. 62, e202300481 (2023).
Vaidya, S. D., Toenjes, S. T., Yamamoto, N., Maddox, S. M. & Gustafson, J. L. Catalytic atroposelective synthesis of N‑aryl quinoid compounds. J. Am. Chem. Soc. 142, 2198–2203 (2020).
Zhu, D., Yu, L., Luo, H.-Y., Xue, X.-S. & Chen, Z.-M. Atroposelective electrophilic sulfenylation of N-aryl aminoquinone derivatives catalyzed by chiral SPINOL-derived sulfide. Angew. Chem. Int. Ed. 61, e202211782 (2022).
Iorio, N. D., Filippini, G., Mazzanti, A., Righi, P. & Bencivenni, G. Controlling the C(sp3)-C(sp2) axial conformation in the enantioselective Friedel-Crafts-type alkylation of β‑naphthols with inden-1-ones. Org. Lett. 19, 6692–6695 (2017).
Wu, X. et al. Catalyst control over sixfold stereogenicity. Nat. Catal. 4, 457–462 (2021). Conformationally stable atropisomers with high-order stereogenicities are attained.
Schmidt, T. A., Schumann, S., Ostertag, A. & Sparr, C. Catalyst control over threefold stereogenicity: selective synthesis of atropisomeric sulfones with stereogenic C-S axes. Angew. Chem. Int. Ed. 62, e202302084 (2023).
Bertuzzi, G. et al. Organocatalytic enantioselective construction of conformationally stable C(sp2)-C(sp3) atropisomers. J. Am. Chem. Soc. 144, 1056–1065 (2022).
Bao, X., Rodriguez, J. & Bonne, D. Enantioselective synthesis of atropisomers with multiple stereogenic axes. Angew. Chem. Int. Ed. 59, 12623–12634 (2020).
Lotter, D., Neuburger, M., Rickhaus, M., Häussinger, D. & Sparr, C. Stereoselective arene-forming aldol condensation: synthesis of configurationally stable oligo-1,2-naphthylenes. Angew. Chem. Int. Ed. 55, 2920–2923 (2016).
Moser, D. & Sparr, C. Synthesis of atropisomeric two-axis systems by the catalyst-controlled syn- and anti-selective arene-forming aldol condensation. Angew. Chem. Int. Ed. 61, e202202548 (2022).
Beleh, O. M., Miller, E., Toste, F. D. & Miller, S. J. Catalytic dynamic kinetic resolutions in tandem to construct two-axis terphenyl atropisomers. J. Am. Chem. Soc. 142, 16461–16470 (2020).
Bao, X., Rodriguez, J. & Bonne, D. Bidirectional enantioselective synthesis of bis-benzofuran atropisomeric oligoarenes featuring two distal C-C stereogenic axes. Chem. Sci. 11, 403–408 (2020).
Luc, A. & Wencel-Delord, J. One reaction—double stereoinduction: C-H activation as a privileged route towards complex atropisomeric molecules. Chem. Commun. 59, 8159–8167 (2023).
Luc, A. et al. Double cobalt-catalyzed atroposelective C-H activation: one-step synthesis of atropisomeric indoles bearing vicinal C-C and C-N diaxes. Chem. Catal. 3, 100765 (2023).
Wang, B.-J. et al. Single-step synthesis of atropisomers with vicinal C-C and C-N diaxes by cobalt-catalyzed atroposelective C-H annulation. Angew. Chem. Int. Ed. 61, e202208912 (2022).
Pummerer, R., Prell, E. & Rieche, A. Darstellung von binaphthylendioxyd. Ber. Dtsch. Chem. Ges. 59, 2159–2161 (1926).
Miyashita, A. et al. Synthesis of 2,2′-bis(diphenylphosphino)-1,1′-binaphthyl (BINAP), an atropisomeric chiral bis(triaryl)phosphine, and its use in the rhodium(I)-catalyzed asymmetric hydrogenation of α-(acylamino)acrylic acids. J. Am. Chem. Soc. 102, 7932–7934 (1980).
Alcock, N. W., Brown, J. M. & Hulmes, D. I. Synthesis and resolution of 1-(2-diphenylphosphino-1-naphthyl)isoquinoline; a P-N chelating ligand for asymmetric catalysis. Tetrahedron: Asymmetry 4, 743–756 (1993).
Hulst, R., de Vries, N. K. & Feringa, B. L. α-Phenylethylamine based chiral phospholidines; new agents for the determination of the enantiomeric excess of chiral alcohols, amines and thiols by means of 31P NMR. Tetrahedron: Asymmetry 5, 699–708 (1994).
Ooi, T., Kameda, M. & Maruoka, K. Molecular design of a C2-symmetric chiral phase-transfer catalyst for practical asymmetric synthesis of α-amino acids. J. Am. Chem. Soc. 121, 6519–6520 (1999).
Nakashima, D. & Yamamoto, H. Design of chiral N-triflyl phosphoramide as a strong chiral Brønsted acid and its applicationto asymmetric Diels-Alder reaction. J. Am. Chem. Soc. 128, 9626–9627 (2006).
Kaib, P. S. J., Schreyer, L., Lee, S., Properzi, R. & List, B. Extremely active organocatalysts enable a highly enantioselective addition of allyltrimethylsilane to aldehydes. Angew. Chem. Int. Ed. 55, 13200–13203 (2016).
Acknowledgements
We are grateful for financial support from the National Natural Science Foundation of China (21825105, 22231004, 22271135 and 22301125), the National Key R&D Program of China (2022YFA1503703 and 2021YFF0701604), Guangdong Innovative Program (2019BT02Y335) and Shenzhen Science and Technology Program (KQTD20210811090112004, JCYJ20210324120205016 and JCYJ20210324105005015). We appreciate assistance from SUSTech Core Research Facilities.
Author information
Authors and Affiliations
Contributions
B.T. and W.-Y.D. supervised the study. S.-H.X. and W.-Y.D. conducted the literature search. B.T., S.-H.X., W.-Y.D. and Y.-B.W. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xiang, SH., Ding, WY., Wang, YB. et al. Catalytic atroposelective synthesis. Nat Catal (2024). https://doi.org/10.1038/s41929-024-01138-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41929-024-01138-z