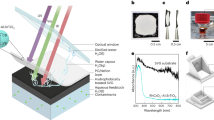
Abstract
With the goal of achieving large-scale H2 production from renewable resources, water splitting into H2 and O2 using semiconductor photocatalysts (sometimes called artificial photosynthesis) has been studied for five decades. Unfortunately, the lack of rigour and reproducibility in the data collection and analysis of experimental results has hindered progress in the field. This Primer provides a comprehensive overview of proper characterization and evaluation of photocatalysts for overall water splitting. In particular, the Primer covers various pitfalls in photocatalysis research, best practices for reproducibility and reliable methods for conducting rigorous experiments. The recommendations are intended to reduce false positives in the literature and to promote progress towards a practical technology for producing H2 from water by using sunlight.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Bolton, J. R., Strickler, S. J. & Connolly, J. S. Limiting and realizable efficiencies of solar photolysis of water. Nature 316, 495–500 (1985). To our knowledge, this is the first paper to quantify the theoretical efficiency limits of solar water splitting.
Fujishima, A. & Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 238, 37–38 (1972). This pioneering work discusses photo-assisted electrolysis of water.
Schrauzer, G. N. & Guth, T. D. Photolysis of water and photoreduction of nitrogen on titanium dioxide. J. Am. Chem. Soc. 99, 7189–7193 (1977).
Domen, K., Naito, S., Soma, M., Onishi, T. & Tamaru, K. Photocatalytic decomposition of water vapour on an NiO–SrTiO3 catalyst. J. Chem. Soc. Chem. Commun. https://doi.org/10.1039/C39800000543 (1980).
Lehn, J. M., Sauvage, J. P. & Ziessel, R. Photochemical water splitting continuous generation of hydrogen and oxygen by irradiation of aqueous suspensions of metal loaded strontium titanate. Nouv. J. Chim. 4, 623–627 (1980).
Sato, S. & White, J. M. Photodecomposition of water over Pt/TiO2 catalysts. Chem. Phys. Lett. 72, 83–86 (1980).
Kudo, A. & Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 38, 253–278 (2009).
Wang, Q. & Domen, K. Particulate photocatalysts for light-driven water splitting: mechanisms, challenges, and design strategies. Chem. Rev. 120, 919–985 (2020).
Takata, T. et al. Photocatalytic water splitting with a quantum efficiency of almost unity. Nature 581, 411–414 (2020). This contribution achieves a milestone in photocatalytic water splitting with a quantum yield of almost unity.
Scaife, D. E. Oxide semiconductors in photoelectrochemical conversion of solar energy. Sol. Energy 25, 41–54 (1980).
Gueymard, C. SMARTS2, A Simple Model of the Atmospheric Radiative Transfer of Sunshine: Algorithms and Performance Assessment (Florida Solar Energy Center/Univ. of Central Florida, 1995).
James, B. D., Baum, G. N., Perez, J. & Baum, K. N. Technoeconomic Analysis of Photoelectrochemical (PEC) Hydrogen Production (Directed Technologies, 2009).
Abe, R. Development of a new system for photocatalytic water splitting into H2 and O2 under visible light irradiation. Bull. Chem. Soc. Jpn. 84, 1000–1030 (2011).
Maeda, K. Z-Scheme water splitting using two different semiconductor photocatalysts. ACS Catal. 3, 1486–1503 (2013).
Wang, Y. et al. Mimicking natural photosynthesis: solar to renewable H2 fuel synthesis by Z-scheme water splitting systems. Chem. Rev. 118, 5201–5241 (2018).
Wang, H. et al. Semiconductor heterojunction photocatalysts: design, construction, and photocatalytic performances. Chem. Soc. Rev. 43, 5234–5244 (2014).
Moniz, S. J. A., Shevlin, S. A., Martin, D. J., Guo, Z.-X. & Tang, J. Visible-light driven heterojunction photocatalysts for water splitting—a critical review. Energy Environ. Sci. 8, 731–759 (2015).
Xu, Q., Zhang, L., Cheng, B., Fan, J. & Yu, J. S-Scheme heterojunction photocatalyst. Chem 6, 1543–1559 (2020).
Maeda, K. & Domen, K. Photocatalytic water splitting: recent progress and future challenges. J. Phys. Chem. Lett. 1, 2655–2661 (2010).
Maeda, K. Photocatalytic water splitting using semiconductor particles: history and recent developments. J. Photochem. Photobiol. C. 12, 237–268 (2011).
Hisatomi, T., Kubota, J. & Domen, K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 43, 7520–7535 (2014).
Konta, R., Ishii, T., Kato, H. & Kudo, A. Photocatalytic activities of noble metal ion doped SrTiO3 under visible light irradiation. J. Phys. Chem. B 108, 8992–8995 (2004).
Asai, R. et al. A visible light responsive rhodium and antimony-codoped SrTiO3 powdered photocatalyst loaded with an IrO2 cocatalyst for solar water splitting. Chem. Commun. 50, 2543–2546 (2014).
Nakada, A. et al. Solar-driven Z-scheme water splitting using tantalum/nitrogen co-doped rutile titania nanorod as an oxygen evolution photocatalyst. J. Mater. Chem. A 5, 11710–11719 (2017).
Nishioka, S. et al. Enhanced water splitting through two-step photoexcitation by sunlight using tantalum/nitrogen-codoped rutile titania as a water oxidation photocatalyst. Sustain. Energy Fuels 3, 2337–2346 (2019).
Miyoshi, A. et al. Nitrogen/fluorine-codoped rutile titania as a stable oxygen-evolution photocatalyst for solar-driven Z-scheme water splitting. Sustain. Energy Fuels 2, 2025–2035 (2018).
Maeda, K. & Domen, K. New non-oxide photocatalysts designed for overall water splitting under visible light. J. Phys. Chem. C 111, 7851–7861 (2007).
Kageyama, H. et al. Expanding frontiers in materials chemistry and physics with multiple anions. Nat. Commun. 9, 772 (2018).
Maeda, K. et al. Recent progress on mixed-anion materials for energy applications. Bull. Chem. Soc. Jpn 95, 26–37 (2022).
Miyoshi, A. & Maeda, K. Recent progress in mixed‐anion materials for solar fuel production. Sol. RRL 5, 521 (2020).
Maeda, K. (Oxy)Nitrides with d0-electronic configuration as photocatalysts and photoanodes that operate under a wide range of visible light for overall water splitting. Phys. Chem. Chem. Phys. 15, 10537–10548 (2013).
Maeda, K. et al. Photocatalyst releasing hydrogen from water. Nature 440, 295 (2006).
Maeda, K., Lu, D. & Domen, K. Direct water splitting into hydrogen and oxygen under visible light by using modified TaON photocatalysts with d0 electronic configuration. Chem. Eur. J. 19, 4986–4991 (2013).
Wang, Q. et al. Oxysulfide photocatalyst for visible-light-driven overall water splitting. Nat. Mater. 18, 827–832 (2019).
Oshima, T. et al. An artificial Z-scheme constructed from dye-sensitized metal oxide nanosheets for visible light-driven overall water splitting. J. Am. Chem. Soc. 142, 8412–8420 (2020).
Tanaka, A., Teramura, K., Hosokawa, S., Kominami, H. & Tanaka, T. Visible light-induced water splitting in an aqueous suspension of a plasmonic Au/TiO2 photocatalyst with metal co-catalysts. Chem. Sci. 8, 2574–2580 (2017).
Wang, X. et al. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 8, 76–80 (2009). To our knowledge, this work is the first report of visible-light H2/O2 evolution using an organic semiconductor.
Zhang, G., Lan, Z. A., Lin, L., Lin, S. & Wang, X. Overall water splitting by Pt/g-C3N4 photocatalysts without using sacrificial agents. Chem. Sci. 7, 3062–3066 (2016).
Nishioka, S., Shibata, K., Miseki, Y., Sayama, K. & Maeda, K. Visible-light-driven nonsacrificial hydrogen evolution by modified carbon nitride photocatalysts. Chin. J. Catal. 43, 2316–2320 (2022).
Zhao, G., Huang, X., Fina, F., Zhang, G. & Irvine, J. T. S. Facile structure design based on C3N4 for mediator-free Z-scheme water splitting under visible light. Catal. Sci. Technol. 5, 3416–3422 (2015).
Martin, D. J., Reardon, P. J., Moniz, S. J. & Tang, J. Visible light-driven pure water splitting by a nature-inspired organic semiconductor-based system. J. Am. Chem. Soc. 136, 12568–12571 (2014).
Fabian, D. M. et al. Particle suspension reactors and materials for solar-driven water splitting. Energy Environ. Sci. 8, 2825–2850 (2015).
Pinaud, B. A. et al. Technical and economic feasibility of centralized facilities for solar hydrogen production via photocatalysis and photoelectrochemistry. Energy Environ. Sci. 6, 1983–2002 (2013).
Zhao, Z. et al. Electronic structure basis for enhanced overall water splitting photocatalysis with aluminum doped SrTiO3 in natural sunlight. Energy Environ. Sci. 12, 1385–1395 (2019). This work explains how aliovalent dopants remove lifetime killers in SrTiO3.
Osterloh, F. E. Photocatalysis versus photosynthesis: a sensitivity analysis of devices for solar energy conversion and chemical transformations. ACS Energy Lett. 2, 445–453 (2017). This perspective explains how the thermodynamics of the photocatalytic reaction dictate the photocatalyst design.
Ohno, T., Bai, L., Hisatomi, T., Maeda, K. & Domen, K. Photocatalytic water splitting using modified GaN:ZnO solid solution under visible light: long-time operation and regeneration of activity. J. Am. Chem. Soc. 134, 8254–8259 (2012).
Goto, Y. et al. A particulate photocatalyst water-splitting panel for large-scale solar hydrogen generation. Joule 2, 509–520 (2018).
Schröder, M. et al. Hydrogen evolution reaction in a large-scale reactor using a carbon nitride photocatalyst under natural sunlight irradiation. Energy Technol. 3, 1014–1017 (2015).
Hisatomi, T., Maeda, K., Takanabe, K., Kubota, J. & Domen, K. Aspects of the water splitting mechanism on (Ga1-xZnx)(N1-xOx) photocatalyst modified with Rh2-yCryO3 cocatalyst. J. Phys. Chem. C 113, 21458–21466 (2009).
Nishioka, S. et al. A zinc-based oxysulfide photocatalyst SrZn2S2O capable of reducing and oxidizing water. Dalton Trans. 48, 15778–15781 (2019).
Maeda, K. et al. GaN:ZnO solid solution as a photocatalyst for visible-light-driven overall water splitting. J. Am. Chem. Soc. 127, 8286–8287 (2005). To our knowledge, this work is the first report of reproducible water splitting into H2 and O2 under visible light.
Schröder, V., Emonts, B., Janßen, H. & Schulze, H. P. Explosion limits of hydrogen/oxygen mixtures at initial pressures up to 200 bar. Chem. Eng. Technol. 27, 847–851 (2004).
Zhou, P., Yu, J. & Jaroniec, M. All-solid-state Z-scheme photocatalytic systems. Adv. Mater. 26, 4920–4935 (2014).
Schneider, J. & Bahnemann, D. W. Undesired role of sacrificial reagents in photocatalysis. J. Phys. Chem. Lett. 4, 3479–3483 (2013).
Wang, J., Zhao, J. & Osterloh, F. E. Photochemical charge transfer observed in nanoscale hydrogen evolving photocatalysts using surface photovoltage spectroscopy. Energy Environ. Sci. 8, 2970–2976 (2015).
Wang, J. & Osterloh, F. E. Limiting factors for photochemical charge separation in BiVO4/Co3O4, a highly active photocatalyst for water oxidation in sunlight. J. Mater. Chem. A 2, 9405–9411 (2014).
Tabata, S., Ohnishi, H., Yagasaki, M., Ippommatsu, M. & Domen, K. Light-intensity dependence in photocatalytic decomposition of water over K4Nb6O17 catalyst. Catal. Lett. 28, 417–422 (1994).
Ikeda, S. et al. Mechano-catalysis — a novel method for overall water splitting. Phys. Chem. Chem. Phys. 1, 4485–4491 (1999).
Kato, H. & Kudo, A. Water splitting into H2 and O2 on alkali tantalate photocatalysts ATaO3 (A = Li, Na, and K). J. Phys. Chem. B 105, 4285–4292 (2001).
Schwarze, M. et al. Quantification of photocatalytic hydrogen evolution. Phys. Chem. Chem. Phys. 15, 3466–3472 (2013).
Serpone, N. Relative photonic efficiencies and quantum yields in heterogeneous photocatalysis. J. Photochem. Photobiol. A 104, 1–12 (1997).
Maeda, K. et al. Efficient overall water splitting under visible-light irradiation on (Ga1-xZnx)(N1-xOx) dispersed with Rh–Cr mixed-oxide nanoparticles: effect of reaction conditions on photocatalytic activity. J. Phys. Chem. B 110, 13107–13112 (2006).
Abe, R., Sayama, K. & Sugihara, H. Development of new photocatalytic water splitting into H2 and O2 using two different semiconductor photocatalysts and a shuttle redox mediator IO3−/I−. J. Phys. Chem. B 109, 16052–16061 (2005).
Wang, Q. et al. Particulate photocatalyst sheets based on carbon conductor layer for efficient Z-scheme pure-water splitting at ambient pressure. J. Am. Chem. Soc. 139, 1675–1683 (2017).
Hisatomi, T., Minegishi, T. & Domen, K. Kinetic assessment and numerical modeling of photocatalytic water splitting toward efficient solar hydrogen production. Bull. Chem. Soc. Jpn. 85, 647–655 (2012).
Herrmann, J.-M. Heterogeneous photocatalysis: fundamentals and applications to the removal of various types of aqueous pollutants. Catal. Today 53, 115–129 (1999).
Maeda, K. et al. Photocatalytic hydrogen evolution from hexaniobate nanoscrolls and calcium niobate nanosheets sensitized by ruthenium(II) bipyridyl complexes. J. Phys. Chem. C. 113, 7962–7969 (2009).
Doménech, J. & Prieto, A. Stability of ZnO particles in aqueous suspensions under UV illumination. J. Phys. Chem. 90, 1123–1126 (1986).
Oshima, T., Lu, D., Ishitani, O. & Maeda, K. Intercalation of highly dispersed metal nanoclusters into a layered metal oxide for photocatalytic overall water splitting. Angew. Chem. Int. Ed. 54, 2698–2702 (2015).
Oshima, T., Yokoi, T., Eguchi, M. & Maeda, K. Synthesis and photocatalytic activity of K2CaNaNb3O10, a new Ruddlesden–Popper phase layered perovskite. Dalton Trans. 46, 10594–10601 (2017).
Maeda, K. et al. Characterization of Rh–Cr mixed-oxide nanoparticles dispersed on (Ga1-xZnx)(N1-xOx) as a cocatalyst for visible-light-driven overall water splitting. J. Phys. Chem. B 110, 13753–13758 (2006).
Kronik, L. & Shapira, Y. Surface photovoltage spectroscopy of semiconductor structures: at the crossroads of physics, chemistry and electrical engineering. Surf. Interface Anal. 31, 954–965 (2001).
Melo, M. A. Jr et al. Surface photovoltage measurements on a particle tandem photocatalyst for overall water splitting. Nano Lett. 18, 805–810 (2018).
Cheng, Y. et al. Effect of charge selective contacts on the quasi Fermi level splitting of CuGa3Se5 thin film photocathodes for hydrogen evolution and methylviologen reduction. EES Catal 1, 74–83 (2023).
Ma, L., Liu, M., Jing, D. & Guo, L. Photocatalytic hydrogen production over CdS: effects of reaction atmosphere studied by in situ Raman spectroscopy. J. Mater. Chem. A 3, 5701–5707 (2015).
Mu, C. et al. In situ characterization techniques applied in photocatalysis: a review. Adv. Mater. Interfaces 10, 2201842 (2022).
Nakato, Y., Ueda, T., Egi, Y. & Tsubomura, H. Decomposition potentials of crystalline silicon as related to the photocurrent stability of p–n junction silicon semiconductor electrodes. J. Electrochem. Soc. 134, 353–358 (1987).
Maeda, K., Abe, R. & Domen, K. Role and function of ruthenium species as promoters with TaON-based photocatalysts for oxygen evolution in two-step water splitting under visible light. J. Phys. Chem. C 115, 3057–3064 (2011).
Xu, P., Milstein, T. J. & Mallouk, T. E. Flat-band potentials of molecularly thin metal oxide nanosheets. ACS Appl. Mater. Interfaces 8, 11539–11547 (2016).
Han, R., Melo, M. A., Zhao, Z., Wu, Z. & Osterloh, F. E. Light intensity dependence of photochemical charge separation in the BiVO4/Ru–SrTiO3:Rh direct contact tandem photocatalyst for overall water splitting. J. Phys. Chem. C. 124, 9724–9733 (2020).
Jia, Q., Iwase, A. & Kudo, A. BiVO4–Ru/SrTiO3:Rh composite Z-scheme photocatalyst for solar water splitting. Chem. Sci. 5, 1513–1519 (2014).
Sasaki, Y., Nemoto, H., Saito, K. & Kudo, A. Solar water splitting using powdered photocatalysts driven by Z-schematic interparticle electron transfer without an electron mediator. J. Phys. Chem. C 113, 17536–17542 (2009).
Nishiyama, H. et al. Photocatalytic solar hydrogen production from water on a 100-m2 scale. Nature 598, 304–307 (2021).
Wang, Q. et al. Scalable water splitting on particulate photocatalyst sheets with a solar-to-hydrogen energy conversion efficiency exceeding 1%. Nat. Mater. 15, 611–615 (2016). This work achieves >1% STH conversion efficiency through photocatalytic water splitting.
Ji, S. M. et al. Photocatalytic hydrogen production from natural seawater. J. Photochem. Photobiol. A 189, 141–144 (2007).
Maeda, K., Masuda, H. & Domen, K. Effect of electrolyte addition on activity of (Ga1−xZnx)(N1−xOx) photocatalyst for overall water splitting under visible light. Catal. Today 147, 173–178 (2009).
Sayama, K. Production of high-value-added chemicals on oxide semiconductor photoanodes under visible light for solar chemical-conversion processes. ACS Energy Lett. 3, 1093–1101 (2018).
Guan, X. et al. Efficient unassisted overall photocatalytic seawater splitting on GaN-based nanowire arrays. J. Phys. Chem. C. 122, 13797–13802 (2018).
Maeda, K. & Domen, K. Development of novel photocatalyst and cocatalyst materials for water splitting under visible light. Bull. Chem. Soc. Jpn. 89, 627–648 (2016).
Maeda, K. Metal-complex/semiconductor hybrid photocatalysts and photoelectrodes for CO2 reduction driven by visible light. Adv. Mater. 31, e1808205 (2019).
Nakada, A., Kumagai, H., Robert, M., Ishitani, O. & Maeda, K. Molecule/semiconductor hybrid materials for visible-light CO2 reduction: design principles and interfacial engineering. Acc. Chem. Res. 2, 458–470 (2021).
Nakada, A. et al. Effects of interfacial electron transfer in metal complex–semiconductor hybrid photocatalysts on Z-scheme CO2 reduction under visible light. ACS Catal. 8, 9744–9754 (2018).
Bak, T., Li, W., Nowotny, J., Atanacio, A. J. & Davis, J. Photocatalytic properties of TiO2: evidence of the key role of surface active sites in water oxidation. J. Phys. Chem. A 119, 9465–9473 (2015).
Nowotny, J. et al. Defect chemistry and defect engineering of TiO2-based semiconductors for solar energy conversion. Chem. Soc. Rev. 44, 8424–8442 (2015).
Nishioka, S. et al. Homogeneous electron doping into nonstoichiometric strontium titanate improves its photocatalytic activity for hydrogen and oxygen evolution. ACS Catal. 8, 7190–7200 (2018).
Vequizo, J. J. M. et al. Crucial impact of reduction on the photocarrier dynamics of SrTiO3 powders studied by transient absorption spectroscopy. J. Mater. Chem. A 7, 26139–26146 (2019).
Ohtani, B. & Takashima, M. Happy photocatalysts and unhappy photocatalysts: electron trap-distribution analysis for metal oxide-sample identification. Catal. Sci. Technol. 12, 354–359 (2022).
Ellis, A. B., Kaiser, S. W., Bolts, J. M. & Wrighton, M. S. Study of n-type semiconducting cadmium chalcogenide-based photoelectrochemical cells employing polychalcogenide electrolytes. J. Am. Chem. Soc. 99, 2839–2848 (1977).
Ishikawa, A. et al. Oxysulfide Sm2Ti2S2O5 as a stable photocatalyst for water oxidation and reduction under visible light irradiation (λ ≦ 650 nm). J. Am. Chem. Soc. 124, 13547–13553 (2002).
Lyu, H. et al. An Al-doped SrTiO3 photocatalyst maintaining sunlight-driven overall water splitting activity for over 1000 h of constant illumination. Chem. Sci. 10, 3196–3201 (2019).
Nandal, V. et al. Unveiling charge dynamics of visible light absorbing oxysulfide for efficient overall water splitting. Nat. Commun. 12, 7055 (2021).
Li, H. et al. One-step excitation overall water splitting over a modified Mg-doped BaTaO2N photocatalyst. ACS Catal. 12, 10179–10185 (2022).
Kato, H., Sasaki, Y., Shirakura, N. & Kudo, A. Synthesis of highly active rhodium-doped SrTiO3 powders in Z-scheme systems for visible-light-driven photocatalytic overall water splitting. J. Mater. Chem. A 1, 12327–12333 (2013).
Nishioka, S. et al. Surface-modified, dye-sensitized niobate nanosheets enabling an efficient solar-driven Z-scheme for overall water splitting. Sci. Adv. 8, eadc9115 (2022).
Abe, R., Sayama, K., Domen, K. & Arakawa, H. A new type of water splitting system composed of two dierent TiO2 photocatalysts (anatase, rutile) and a IO3–/I– shuttle redox mediator. Chem. Phys. Lett. 344, 339–344 (2001).
Abe, R., Sayama, K. & Arakawa, H. Significant effect of iodide addition on water splitting into H2 and O2 over Pt-loaded TiO2 photocatalyst: suppression of backward reaction. Chem. Phys. Lett. 371, 360–364 (2003).
Kim, W., Tachikawa, T., Majima, T. & Choi, W. Photocatalysis of dye-sensitized TiO2 nanoparticles with thin overcoat of Al2O3: enhanced activity for H2 production and dechlorination of CCl4. J. Phys. Chem. C. 113, 10603–10609 (2009).
Nishioka, S. et al. Excited carrier dynamics in a dye-sensitized niobate nanosheet photocatalyst for visible-light hydrogen evolution. ACS Catal. 11, 659–669 (2021).
Saupe, G. B., Mallouk, T. E., Kim, W. & Schmehl, R. H. Visible light photolysis of hydrogen iodide using sensitized layered metal oxide semiconductors: the role of surface chemical modification in controlling back electron transfer reactions. J. Phys. Chem. B 101, 2508–2513 (1997).
Acknowledgements
S.N. acknowledges support by a Grant-in-Aid for Research Activity Start-up (JP21K20555). F.E.O. acknowledges support from the US Department of Energy, Office of Science, Office of Basic Energy Sciences (Award Number DOE-SC0015329). X.W. acknowledges support from the National Natural Science Foundation of China (22032002 and U1905214). T.E.M. acknowledges support from the Office of Basic Energy Sciences, Division of Chemical Sciences, Geosciences, and Energy Biosciences, Department of Energy (contract DE-SC0019781). K.M. acknowledges financial support by a Grant-in-Aid for Scientific Research (B) (JP22H01862), a Grant-in-Aid for Transformative Research Areas (A) “Supra-ceramics” (JP22H05148) and a Core-to-Core Program (JPJSCCA20200004) (JSPS).
Author information
Authors and Affiliations
Contributions
Introduction (S.N., F.E.O., X.W., T.E.M. and K.M.); Experimentation (S.N., F.E.O., X.W., T.E.M. and K.M.); Results (S.N., F.E.O., X.W., T.E.M. and K.M.); Applications (S.N., F.E.O., X.W., T.E.M. and K.M.); Reproducibility and data deposition (S.N., F.E.O., X.W., T.E.M. and K.M.); Limitations and optimizations (S.N., F.E.O., X.W., T.E.M. and K.M.); Outlook (S.N., F.E.O., X.W., T.E.M. and K.M.); Overview of the Primer (S.N., F.E.O., X.W., T.E.M. and K.M.).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Methods Primers thanks Yingtang Zhou, Ling Piao, Kamalakannan Kailasam and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Back reactions
-
Reactions that can lower the efficiency of photocatalytic water splitting (for example, H2 + ½O2 → H2O).
- Black H2
-
H2 that is produced from bituminous coal via steam reforming, which produces high quantities of CO2.
- Conduction band (CB) minimum
-
The energy region in a semiconductor that corresponds to empty antibonding orbitals and that conducts electrons when present from excitation.
- Foreign element
-
An impurity added to semiconductors to control their characteristics (for example, a metal cation that can contribute d electrons, or an anion that is less electronegative than oxygen).
- Grey H2
-
H2 that is produced from natural gas or methane via steam reforming, which produces lower quantities of CO2.
- Merry-go-round system
-
Samples placed equidistant from the light source are rotated around the light source, and each sample is illuminated with light of the same intensity.
- Sacrificial reagents
-
Fast electron donors or acceptors that may be irreversibly decomposed after reaction and that modify the energetics and rate of the photocatalytic reaction.
- Standard hydrogen electrode
-
(SHE). The hydrogen electrode when the activities of hydrogen gas and proton are both 1.
- Valence band (VB) maximum
-
The energy region in a semiconductor that corresponds to filled bonding orbitals and that conducts holes when present from excitation.
- Z-Scheme
-
A two-step photochemical reaction taking place in plants to utilize lower energy photons and generate sufficient potential to drive photosynthetic reactions, The electron transfer pathway resembles the letter Z.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nishioka, S., Osterloh, F.E., Wang, X. et al. Photocatalytic water splitting. Nat Rev Methods Primers 3, 42 (2023). https://doi.org/10.1038/s43586-023-00226-x
Accepted:
Published:
DOI: https://doi.org/10.1038/s43586-023-00226-x
This article is cited by
-
Exploiting hot electrons from a plasmon nanohybrid system for the photoelectroreduction of CO2
Communications Chemistry (2024)
-
Efficient hydrogen production and electricity generation in solar-driven single-photoelectrode photocatalytic fuel cell
Catalysis Letters (2024)
-
TiO2-based S-scheme photocatalysts for solar energy conversion and environmental remediation
Science China Materials (2024)
-
A leap towards solar-powered water and energy solutions
Nature Water (2023)