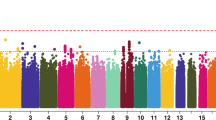
Abstract
Maternal depressive symptoms are highly prevalent and can negatively impact affected individuals and family members. Understanding etiological influences on maternal depression, such as genetic liability, is key to inform treatment and prevention efforts. Here we quantified direct and indirect genetic effects (that is, when genetic variants in other individuals influence risk of maternal depression through the environment) from partners and offspring on maternal depressive symptoms at multiple time points using genome-wide complex trait analysis with parent–offspring trios. We used data from the Norwegian Mother, Father and Child cohort study, including up to 21,000 genotyped parent–offspring trios. Models with indirect genetic effects had best fit at three out of five time points (3, 5 and 8 years after birth). The variance in maternal depressive symptoms explained by direct genetic effects ranged from 5% to 14%, whereas indirect genetic effects explained 0–14% of variance across time points. Heritable traits in family members contribute to maternal depressive symptoms through the environment at several time points after birth.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$59.00 per year
only $4.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
MoBa data can be accessed by application to the Regional Committee for Medical and Health 494 Research Ethics in Norway and MoBa (https://www.fhi.no/en/ch/studies/moba/for-forskere-artikler/research-and-data-access/). The consent given by the participants does not open for storage of data on an individual level in repositories or journals.
Code availability
The code used in this study is available upon request from the first author. Example code for fitting variance-component models structured according to relationship matrices with VCModels.jl is provided at: https://github.com/espenmei/VCModels.jl.
References
Gavin, N. I. et al. Perinatal depression: a systematic review of prevalence and incidence. Obstet. Gynecol. 106, 1071–1083 (2005).
O’Hara, M. W. & McCabe, J. E. Postpartum depression: current status and future directions. Annu. Rev. Clin. Psychol. 9, 379–407 (2013).
O’Hara, M. W. & Swain, A. M. Rates and risk of postpartum depression—a meta-analysis. Int. Rev. Psychiatry 8, 37–54 (1996).
Horwitz, S. M., Briggs-Gowan, M. J., Storfer-Isser, A. & Carter, A. S. Prevalence, correlates, and persistence of maternal depression. J. Womens Health 16, 678–691 (2007).
Horwitz, S. M., Briggs-Gowan, M. J., Storfer-Isser, A. & Carter, A. S. Persistence of maternal depressive symptoms throughout the early years of childhood. J. Womens Health 18, 637–645 (2009).
Gjerde, L. C. et al. Maternal perinatal and concurrent depressive symptoms and child behavior problems: a sibling comparison study. J. Child Psychol. Psychiatry 58, 779–786 (2017).
Gjerde, L. C. et al. Associations between maternal depressive symptoms and risk for offspring early-life psychopathology: the role of genetic and non-genetic mechanisms. Psychol. Med. 51, 441–449 (2021).
Field, T. Postpartum depression effects on early interactions, parenting, and safety practices: a review. Infant Behav. Dev. 33, 1–6 (2010).
Letourneau, N. L. et al. Postpartum depression is a family affair: addressing the impact on mothers, fathers, and children. Issues Ment. Health Nurs. 33, 445–457 (2012).
Lovejoy, M. C., Graczyk, P. A., O’Hare, E. & Neuman, G. Maternal depression and parenting behavior: a meta-analytic review. Clin. Psychol. Rev. 20, 561–592 (2000).
Guintivano, J., Manuck, T. & Meltzer-Brody, S. Predictors of postpartum depression: a comprehensive review of the last decade of evidence. Clin. Obstet. Gynecol. 61, 591–603 (2018).
Polderman, T. J. C. et al. Meta-analysis of the heritability of human traits based on fifty years of twin studies. Nat. Genet. 47, 702–709 (2015).
Sullivan, P. F., Neale, M. C. & Kendler, K. S. Genetic epidemiology of major depression: review and meta-analysis. Am. J. Psychiatry 157, 1552–1562 (2000).
Samuelsen, K., Ystrom, E., Gjerde, L. C. & Eilertsen, E. M. Kind of blue—an evaluation of etiologies for prenatal versus postnatal depression symptoms. J. Affect. Disord. 335, 305–312 (2023).
Treloar, S. A., Martin, N. G., Bucholz, K. K., Madden, P. A. F. & Heath, A. C. Genetic influences on post-natal depressive symptoms: findings from an Australian twin sample. Psychol. Med. 29, 645–654 (1999).
Viktorin, A. et al. Heritability of perinatal depression and genetic overlap With nonperinatal depression. Am. J. Psychiatry 173, 158–165 (2016).
Howard, D. M. et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat. Neurosci. 22, 343–352 (2019).
Levey, D. F. et al. Bi-ancestral depression GWAS in the Million Veteran Program and meta-analysis in >1.2 million individuals highlight new therapeutic directions. Nat. Neurosci. 24, 954–963 (2021).
Wray, N. R. et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat. Genet. 50, 668–681 (2018).
Pilkington, P. D., Milne, L. C., Cairns, K. E., Lewis, J. & Whelan, T. A. Modifiable partner factors associated with perinatal depression and anxiety: a systematic review and meta-analysis. J. Affect. Disord. 178, 165–180 (2015).
Werner, E., Miller, M., Osborne, L. M., Kuzava, S. & Monk, C. Preventing postpartum depression: review and recommendations. Arch. Womens Ment. Health 18, 41–60 (2015).
Austin, M.-P., Hadzi-Pavlovic, D., Leader, L., Saint, K. & Parker, G. Maternal trait anxiety, depression and life event stress in pregnancy: relationships with infant temperament. Early Hum. Dev. 81, 183–190 (2005).
Beck, C. T. Predictors of postpartum depression: an update. Nurs. Res. 50, 275–285 (2001).
Britton, J. R. Infant temperament and maternal anxiety and depressed mood in the early postpartum period. Women Health 51, 55–71 (2011).
McGrath, J. M., Records, K. & Rice, M. Maternal depression and infant temperament characteristics. Infant Behav. Dev. 31, 71–80 (2008).
Ahmadzadeh, Y. I. et al. Anxiety in the family: a genetically informed analysis of transactional associations between mother, father and child anxiety symptoms. J. Child Psychol. Psychiatry 60, 1269–1277 (2019).
McAdams, T. A. et al. The relationship between parental depressive symptoms and offspring psychopathology: evidence from a children-of-twins study and an adoption study. Psychol. Med. 45, 2583–2594 (2015).
Landolt, M. A., Ystrom, E., Stene-Larsen, K., Holmstrøm, H. & Vollrath, M. E. Exploring causal pathways of child behavior and maternal mental health in families with a child with congenital heart disease: a longitudinal study. Psychol. Med. 44, 3421–3433 (2014).
Ystrom, H., Nilsen, W., Hysing, M., Sivertsen, B. & Ystrom, E. Sleep problems in preschoolers and maternal depressive symptoms: an evaluation of mother- and child-driven effects. Dev. Psychol. 53, 2261–2272 (2017).
Kong, A. et al. The nature of nurture: effects of parental genotypes. Science 359, 424–428 (2018).
McAdam, A. G., Garant, D. & Wilson, A. J. in Quantitative Genetics in the Wild 84–103 (Oxford Univ. Press, 2014).
Young, A. I., Benonisdottir, S., Przeworski, M. & Kong, A. Deconstructing the sources of genotype–phenotype associations in humans. Science 365, 1396–1400 (2019).
Fearon, R. M. P. et al. Child-evoked maternal negativity from 9 to 27 months: evidence of gene–environment correlation and its moderation by marital distress. Dev. Psychopathol. 27, 1251–1265 (2015).
Ge, X. et al. The developmental interface between nature and nurture: a mutual influence model of child antisocial behavior and parent behaviors. Dev. Psychol. 32, 574–589 (1996).
Cheesman, R. et al. How important are parents in the development of child anxiety and depression? A genomic analysis of parent-offspring trios in the Norwegian Mother Father and Child cohort study (MoBa). BMC Med. 18, 1–11 (2020).
Eilertsen, E. M. et al. On the importance of parenting in externalizing disorders: an evaluation of indirect genetic effects in families. J. Child Psychol. Psychiatry 63, 1186–1195 (2022).
Howe, L. J. et al. Within-sibship genome-wide association analyses decrease bias in estimates of direct genetic effects. Nat. Genet. 54, 581–592 (2022).
Eilertsen, E. M. et al. Direct and indirect effects of maternal, paternal, and offspring genotypes: trio-GCTA. Behav. Genet. 51, 154–161 (2021).
Yang, J. et al. Common SNPs explain a large proportion of heritability for human height. Nat. Genet. 42, 565–569 (2010).
Yang, J., Lee, S. H., Goddard, M. E. & Visscher, P. M. GCTA: a tool for genome-wide complex trait analysis. Am. J. Hum. Genet. 88, 76–82 (2011).
Yang, J., Zeng, J., Goddard, M. E., Wray, N. R. & Visscher, P. M. Concepts, estimation and interpretation of SNP-based heritability. Nat. Genet. 49, 1304–1310 (2017).
Magnus, P. et al. Cohort profile update: the Norwegian Mother and Child cohort study (MoBa). Int. J. Epidemiol. 45, 382–388 (2016).
Young, A. I. et al. Relatedness disequilibrium regression estimates heritability without environmental bias. Nat. Genet. 50, 1304–1310 (2018).
Cai, N., Choi, K. W. & Fried, E. I. Reviewing the genetics of heterogeneity in depression: operationalizations, manifestations and etiologies. Hum. Mol. Genet. 29, R10–R18 (2020).
Lee, S. H. et al. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat. Genet. 45, 984–994 (2013).
Lubke, G. H. et al. Estimating the genetic variance of major depressive disorder due to all single nucleotide polymorphisms. Biol. Psychiatry 72, 707–709 (2012).
Laurin, C. A., Hottenga, J.-J., Willemsen, G., Boomsma, D. I. & Lubke, G. H. Genetic analyses benefit from using less heterogeneous phenotypes: an illustration with the Hospital Anxiety and Depression Scale (HADS). Genet. Epidemiol. 39, 317–324 (2015).
Bjørndal, L. D., Kendler, K. S., Reichborn-Kjennerud, T. & Ystrom, E. Stressful life events increase the risk of major depressive episodes: a population-based twin study. Psychol. Med. 53, 5194–5202 (2022).
Ayorech, Z. et al. Maternal depression and the polygenic p factor: a family perspective on direct and indirect effects. J. Affect. Disord. 332, 159–167 (2023).
Madigan, S., Wade, M., Plamondon, A. & Jenkins, J. M. Trajectories of maternal depressive symptoms in the early childhood period and family-wide clustering of risk. J. Affect. Disord. 215, 49–55 (2017).
Ystrøm, E. et al. Multiple births and maternal mental health from pregnancy to 5 years after birth: a longitudinal population-based cohort study. Nor. Epidemiol. 24, 203–208 (2014).
Nes, R. B., Røysamb, E., Reichborn-Kjennerud, T., Harris, J. R. & Tambs, K. Symptoms of anxiety and depression in young adults: genetic and environmental influences on stability and change. Twin Res. Hum. Genet. 10, 450–461 (2007).
Nivard, M. G. et al. Stability in symptoms of anxiety and depression as a function of genotype and environment: a longitudinal twin study from ages 3 to 63 years. Psychol. Med. 45, 1039–1049 (2015).
Räsänen, K. & Kruuk, L. E. B. Maternal effects and evolution at ecological time-scales. Funct. Ecol. 21, 408–421 (2007).
Biele, G. et al. Bias from self selection and loss to follow-up in prospective cohort studies. Eur. J. Epidemiol. 34, 927–938 (2019).
Nilsen, R. M. et al. Self-selection and bias in a large prospective pregnancy cohort in Norway. Paediatr. Perinat. Epidemiol. 23, 597–608 (2009).
Cheesman, R., Ayorech, Z., Eilertsen, E. M. & Ystrom, E. Why we need families in genomic research on developmental psychopathology. JCPP Adv. 3, 1–9 (2023).
Demange, P. A. et al. Estimating effects of parents’ cognitive and non-cognitive skills on offspring education using polygenic scores. Nat. Commun. 13, 4801 (2022).
Horwitz, T. B., Balbona, J. V., Paulich, K. N. &Keller, M. C. Evidence of correlations between human partners based on systematic reviews and meta-analyses of 22 traits and UK Biobank analysis of 133 traits. Nat. Hum. Behav. 7, 1568–1583 (2023).
Peyrot, W. J., Robinson, M. R., Penninx, B. W. J. H. & Wray, N. R. Exploring boundaries for the genetic consequences of assortative mating for psychiatric traits. JAMA Psychiatry 73, 1189–1195 (2016).
Eilertsen, E. M. et al. Parental prenatal symptoms of depression and offspring dymptoms of ADHD: a genetically informed intergenerational study. J. Atten. Disord. 25, 1554–1563 (2021).
Torvik, F. A. et al. Modeling assortative mating and genetic similarities between partners, siblings, and in-laws. Nat. Commun. 13, 1–10 (2022).
Ayorech, Z. et al. The structure of psychiatric comorbidity without selection and assortative mating. Transl. Psychiatry (in press).
Sunde, H. F. et al. Genetic similarity between relatives provides evidence on the presence and history of assortative mating. Preprint at bioRxiv https://doi.org/10.1101/2023.06.27.546663 (2023).
Corfield, E. C. et al. The Norwegian Mother, Father, and Child cohort study (MoBa) genotyping data resource: MoBaPsychGen pipeline v.1. Preprint at bioRxiv https://doi.org/10.1101/2022.06.23.496289 (2022).
Zhou, H. et al. OpenMendel: a cooperative programming project for statistical genetics. Hum. Genet. 139, 61–71 (2020).
Hesbacher, P. T., Rickels, K., Morris, R. J., Newman, H. & Rosenfeld, H. Psychiatric illness in family practice. J. Clin. Psychiatry. 41, 6–10 (1980).
Tambs, K. & Røysamb, E. Selection of questions to short-form versions of original psychometric instruments in MoBa. Nor. Epidemiol. 24, 195–201 (2014).
Fink, P., Ørnbøl, E., Hansen, M. S., Søndergaard, L. & De Jonge, P. Detecting mental disorders in general hospitals by the SCL-8 scale. J. Psychosom. Res. 56, 371–375 (2004).
Fink, P. et al. A brief diagnostic screening instrument for mental disturbances in general medical wards. J. Psychosom. Res. 57, 17–24 (2004).
Eaves, L. J., St. Pourcain, B., Smith, G. D., York, T. P. & Evans, D. M. Resolving the effects of maternal and offspring genotype on dyadic outcomes in genome-wide complex trait analysis (‘M-GCTA’). Behav. Genet. 44, 445–455 (2014).
Akaike, H. Factor analysis and AIC. Psychometrika 52, 317–332 (1987).
Dominicus, A., Skrondal, A., Gjessing, H. K., Pedersen, N. L. & Palmgren, J. Likelihood ratio tests in behavioral genetics: problems and solutions. Behav. Genet. 36, 331–340 (2006).
Wu, H. & Neale, M. C. On the likelihood ratio tests in bivariate ACDE models. Psychometrika 78, 441–463 (2013).
Bezanson, J., Edelman, A., Karpinski, S. & Shah, V. B. Julia: A fresh approach to numerical computing. SIAM Rev. 59, 65–98 (2017).
Eilertsen, E. M. VCModels.jl—a Julia package for fitting variance component models with relationship matrices version 0.1.0; https://github.com/espenmei/VCModels.jl (2021).
Acknowledgements
The Research Council of Norway supported R.C., R.B.N., E.R., and T.A.M. (288083); H.A. (274611); A.H. (274611, 336085); L.D.B. (314843) and E.Y. 288083, 262177, 336078, and 331640). The South-Eastern Norway Regional Health Authority supported L.J.H. (2022083 and 2018058) and A.H. (2020022). L.D.B. has received internationalization support from UiO:Life Science. J.R.B. is supported by a Sir Henry Wellcome Postdoctoral Fellowship (215917/Z/19/Z). The positions of T.A.M. and Y.I.A., were funded by a Wellcome Trust Senior Research Fellowship awarded to T.A.M. (220382/Z/20/Z). E.Y. is funded by the European Research Council under the Horizon 2020 (818425) and the Horizon Europa program (101045526). Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union or the European Research Executive Agency. Neither the European Union nor the granting authority can be held responsible for them.
Author information
Authors and Affiliations
Contributions
L.D.B., E.M.E. and E.Y. were responsible for the concept, design and analysis of data. L.D.B., E.M.E., Z.A., R.C., Y.I.A., J.R.B., H.A., L.J.H., T.A.M., A.H., R.B.N., E.R. and E.Y. contributed to the interpretation of the results. Drafting of the manuscript was done by L.D.B., while E.M.E., Z.A., R.C., Y.I.A., J.R.B., H.A., L.J.H., T.A.M., A.H., R.B.N., E.R. and E.Y. provided critical revision of the manuscript for important intellectual content.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Mental Health thanks Justin Tubbs and the other, anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bjørndal, L.D., Eilertsen, E.M., Ayorech, Z. et al. Disentangling direct and indirect genetic effects from partners and offspring on maternal depression using trio-GCTA. Nat. Mental Health 2, 417–425 (2024). https://doi.org/10.1038/s44220-024-00207-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44220-024-00207-3