Featured
-
-
Article |
 The episodic resurgence of highly pathogenic avian influenza H5 virus
The episodic resurgence of highly pathogenic avian influenza H5 virus
Recent resurgences of highly pathogenic avian influenza H5 viruses have different origins and virus ecologies as their epicentres shift and viruses evolve, with changes indicating increased adaptation among domestic birds.
- Ruopeng Xie
- , Kimberly M. Edwards
- & Vijaykrishna Dhanasekaran
-
Article |
 BTN3A3 evasion promotes the zoonotic potential of influenza A viruses
BTN3A3 evasion promotes the zoonotic potential of influenza A viruses
A protein that evolved in primates, BTN3A3, is expressed in human airways and shows antiviral activity against avian IAVs but not against human IAVs.
- Rute Maria Pinto
- , Siddharth Bakshi
- & Massimo Palmarini
-
Article
| Open Access A pan-influenza antibody inhibiting neuraminidase via receptor mimicry
A pan-influenza antibody inhibiting neuraminidase via receptor mimicry
The neuraminidase-targeting monoclonal antibody FNI9 potently inhibits the enzymatic activity of influenza A and B viruses via receptor mimicry.
- Corey Momont
- , Ha V. Dang
- & Matteo Samuele Pizzuto
-
Article |
 Quadrivalent influenza nanoparticle vaccines induce broad protection
Quadrivalent influenza nanoparticle vaccines induce broad protection
A nanoparticle influenza vaccine candidate is shown to induce broad cross-reactive antibody responses in animal models.
- Seyhan Boyoglu-Barnum
- , Daniel Ellis
- & Masaru Kanekiyo
-
Article |
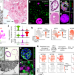 Progenitor identification and SARS-CoV-2 infection in human distal lung organoids
Progenitor identification and SARS-CoV-2 infection in human distal lung organoids
A long-term culture method for organoids derived from single adult human lung cells is used to identify progenitor cells and study SARS-CoV-2 infection.
- Ameen A. Salahudeen
- , Shannon S. Choi
- & Calvin J. Kuo
-
Article |
 Host ANP32A mediates the assembly of the influenza virus replicase
Host ANP32A mediates the assembly of the influenza virus replicase
Structural and biochemical studies of influenza virus RNA polymerase in complex with host acidic nuclear phosphoprotein 32 (ANP32) show how ANP32-mediated polymerase dimerization enables the replication of influenza viral RNA in a host-dependent manner.
- Loïc Carrique
- , Haitian Fan
- & Jonathan M. Grimes
-
Article |
 The native structure of the assembled matrix protein 1 of influenza A virus
The native structure of the assembled matrix protein 1 of influenza A virus
Structures of the assembled matrix protein 1 of influenza A virus in intact virus particles and of oligomers of this protein reconstituted in vitro reveal mechanisms of assembly and disassembly of influenza virus.
- Julia Peukes
- , Xiaoli Xiong
- & John A. G. Briggs
-
Article |
 Structural transitions in influenza haemagglutinin at membrane fusion pH
Structural transitions in influenza haemagglutinin at membrane fusion pH
Cryo-electron microscopy studies of the influenza haemagglutinin glycoprotein at the low pH of host endosomes reveals structural intermediates, offering a dynamic view of how the protein mediates membrane fusion.
- Donald J. Benton
- , Steven J. Gamblin
- & John J. Skehel
-
Letter |
 Structures of influenza A virus RNA polymerase offer insight into viral genome replication
Structures of influenza A virus RNA polymerase offer insight into viral genome replication
Structures of RNA polymerase of human and avian influenza A viruses reveal that the interface of the RNA polymerase dimer is required to initiate viral RNA synthesis in viral genome replication.
- Haitian Fan
- , Alexander P. Walker
- & Ervin Fodor
-
Letter |
 MHC class II proteins mediate cross-species entry of bat influenza viruses
MHC class II proteins mediate cross-species entry of bat influenza viruses
The DR isotype of the human leukocyte antigen of the MHC class II—or its homologues in bats, pigs, mice and chickens—is an essential cell entry determinant for bat influenza A viruses.
- Umut Karakus
- , Thiprampai Thamamongood
- & Silke Stertz
-
Letter |
 Structural basis of an essential interaction between influenza polymerase and Pol II CTD
Structural basis of an essential interaction between influenza polymerase and Pol II CTD
The crystal structure of bat influenza A polymerase bound to a serine-5 phosphorylated peptide mimic from the C-terminal domain of cellular RNA polymerase II shows how the two polymerases are directly coupled and suggests that the interaction site could be targeted for antiviral drug development.
- Maria Lukarska
- , Guillaume Fournier
- & Stephen Cusack
-
Letter |
 Species difference in ANP32A underlies influenza A virus polymerase host restriction
Species difference in ANP32A underlies influenza A virus polymerase host restriction
The host protein ANP32A is shown here to be a species barrier to the function of avian influenza virus polymerase in mammalian cells; the mutation E627K in viral protein PB2, which allows mammalian ANP32 family proteins to support the avian virus polymerase, is known to be associated with increased virulence of avian viruses in mammals.
- Jason S. Long
- , Efstathios S. Giotis
- & Wendy S. Barclay
-
Letter |
 Crystal structure of the RNA-dependent RNA polymerase from influenza C virus
Crystal structure of the RNA-dependent RNA polymerase from influenza C virus
The X-ray crystal structure of influenza C virus polymerase, captured in a closed, pre-activation confirmation, is solved at 3.9 Å resolution; comparison with previous RNA-bound structures reveals large conformational changes associated with RNA binding and activation, and illustrates the notable flexibility of the influenza virus RNA polymerase.
- Narin Hengrung
- , Kamel El Omari
- & Ervin Fodor
-
Letter |
 The soft palate is an important site of adaptation for transmissible influenza viruses
The soft palate is an important site of adaptation for transmissible influenza viruses
Efficient airborne transmission of influenza viruses between humans is associated with use of α2,6-linked sialic acids, not α2,3-linked sialic acids; however, using a loss-of-function approach in which a 2009 pandemic H1N1 influenza virus was engineered to bind α2,3 sialic acids, this study shows in ferrets that the soft palate is an important site for the switch of receptor usage to take place, and reveals that this tissue rapidly selects for transmissible influenza virus with human receptor preference.
- Seema S. Lakdawala
- , Akila Jayaraman
- & Kanta Subbarao
-
Letter |
 Global circulation patterns of seasonal influenza viruses vary with antigenic drift
Global circulation patterns of seasonal influenza viruses vary with antigenic drift
The analysis of more than 9,000 haemagglutinin sequences of human seasonal influenza viruses over a 12-year time period shows that the global circulation patterns of A/H1N1 and B viruses are different from those of the well characterised A/H3N2 viruses; in particular the A/H1N1 and B viruses are shown to persist locally across several seasons and do not display the same degree of global movement as the H3N2 viruses.
- Trevor Bedford
- , Steven Riley
- & Colin A. Russell
-
Letter |
 Dissemination, divergence and establishment of H7N9 influenza viruses in China
Dissemination, divergence and establishment of H7N9 influenza viruses in China
Influenza surveillance over 15 cities across 5 provinces in China from October 2013 to July 2014 shows that the virus has diverged into distinct clades, becoming established in chickens and also disseminating to wider geographic regions.
- Tommy Tsan-Yuk Lam
- , Boping Zhou
- & Huachen Zhu
-
Article |
 Structural insight into cap-snatching and RNA synthesis by influenza polymerase
Structural insight into cap-snatching and RNA synthesis by influenza polymerase
Atomic resolution crystal structures of influenza A and B polymerases are presented; comparison of these structures provides mechanistic insight into influenza polymerase functions, explaining the processes of cap-snatching and cap-dependent priming, which are unique to segmented negative-strand RNA viruses.
- Stefan Reich
- , Delphine Guilligay
- & Stephen Cusack
-
Article |
 Structure of influenza A polymerase bound to the viral RNA promoter
Structure of influenza A polymerase bound to the viral RNA promoter
The crystal structure of the bat-specific influenza A polymerase in complex with the viral RNA promoter is presented, revealing how binding of the 5′ end of the viral RNA is required to activate or enhance the polymerase allosterically.
- Alexander Pflug
- , Delphine Guilligay
- & Stephen Cusack
-
Letter |
 A synchronized global sweep of the internal genes of modern avian influenza virus
A synchronized global sweep of the internal genes of modern avian influenza virus
A local molecular clock approach shows that most genetic diversity in avian influenza virus (AIV) arose in a recent global sweep and that avian strains are the sister group to equine H7N7; most of the 1918 pandemic virus’s genes originated from the resulting western hemispheric AIV lineage.
- Michael Worobey
- , Guan-Zhu Han
- & Andrew Rambaut
-
Letter |
 The genesis and source of the H7N9 influenza viruses causing human infections in China
The genesis and source of the H7N9 influenza viruses causing human infections in China
Evolutionary analyses show that H7 influenza viruses probably transferred from ducks to chickens in China on at least two independent occasions, and that reassortment with H9N2 viruses generated the H7N9 outbreak lineage that recently emerged in humans in China, and a related previously unrecognized H7N7 lineage; these H7N7 viruses are shown to have the ability to infect ferrets, and the current pandemic threat could extend beyond H7N9 viruses.
- Tommy Tsan-Yuk Lam
- , Jia Wang
- & Yi Guan
-
Letter |
 Limited airborne transmission of H7N9 influenza A virus between ferrets
Limited airborne transmission of H7N9 influenza A virus between ferrets
An investigation into the transmissibility of the H7N9 influenza A virus in ferrets finds that although the virus has some determinants associated with human adaptation and transmissibility between mammals, the airborne transmission between ferrets is limited.
- Mathilde Richard
- , Eefje J. A. Schrauwen
- & Ron A. M. Fouchier
-
Letter |
 Characterization of H7N9 influenza A viruses isolated from humans
Characterization of H7N9 influenza A viruses isolated from humans
Here, biological attributes of two early human isolates of the newly emerged H7N9 influenza viruses are characterized: the potential of these viruses to infect and/or transmit within various animal models is discussed, as is their relative sensitivity to neuraminidase inhibitors and experimental polymerase inhibitors compared to an H1N1 pandemic strain.
- Tokiko Watanabe
- , Maki Kiso
- & Yoshihiro Kawaoka
-
Letter |
 Biological features of novel avian influenza A (H7N9) virus
Biological features of novel avian influenza A (H7N9) virus
An initial characterization of the receptor-binding properties of the novel avian influenza A (H7N9) shows that the virus has acquired the ability to bind human receptors while retaining the ability to bind avian receptors; the virus infects epithelial cells in the human lower respiratory tract and type II pneumocytes in the alveoli, and hypercytokinaemia was seen in infected patients.
- Jianfang Zhou
- , Dayan Wang
- & Yuelong Shu
-
Comment |
 Self-censorship is not enough
Self-censorship is not enough
The debate over publishing potentially dangerous research on flu viruses would benefit from a closer look at history, argue David Kaiser and Jonathan D. Moreno.
- David Kaiser
- & Jonathan Moreno
-
News |
 Controversial H5N1 influenza work likely to resume
Controversial H5N1 influenza work likely to resume
International experts hammer out the future of their field at a top-level workshop on how to fund certain types of work on bird flu.
- Brendan Maher
-
Article |
 Cross-neutralization of influenza A viruses mediated by a single antibody loop
Cross-neutralization of influenza A viruses mediated by a single antibody loop
The crystal structure of an influenza antibody that recognizes a small, conserved site in the variable receptor-binding domain of HA is described; this antibody shows broad neutralization across multiple subtypes of influenza A virus through an antibody–antigen interaction dominated by a single heavy-chain complementarity-determining region 3 loop.
- Damian C. Ekiert
- , Arun K. Kashyap
- & Ian A. Wilson
-
News |
 Need for flu surveillance reiterated
Need for flu surveillance reiterated
Study of Korean pigs finds virus with pandemic potential.
- Ed Yong
-
Letter |
 Structural and genetic basis for development of broadly neutralizing influenza antibodies
Structural and genetic basis for development of broadly neutralizing influenza antibodies
The events leading to the generation of broadly neutralizing antibodies to influenza viruses, which may hold the key to developing a universal flu vaccine, are elucidated.
- Daniel Lingwood
- , Patrick M. McTamney
- & Gary J. Nabel
-
News |
 Freeze on mutant-flu research set to thaw
Freeze on mutant-flu research set to thaw
But some fear that if more labs work on the viruses, the risk of accidental release will multiply.
- Declan Butler
-
News Feature |
 Influenza: Five questions on H5N1
Influenza: Five questions on H5N1
Scientists now know that the deadly bird flu virus is capable of causing a human pandemic. That makes tackling the remaining unknowns all the more urgent.
- Ed Yong
-
Perspective |
Engineering H5N1 avian influenza viruses to study human adaptation
Engineering influenza viruses to study human adaptation is a controversial area of research, with opinions diverging over the wisdom of publishing the full results of such studies.
- David M. Morens
- , Kanta Subbarao
- & Jeffery K. Taubenberger
-
Research Highlights |
Towards a single flu vaccine
-
News Feature |
 Bird-flu research: The biosecurity oversight
Bird-flu research: The biosecurity oversight
The fight over mutant flu has thrown the spotlight on a little-known government body that oversees dual-use research. Some are asking if it was up to the task.
- Brendan Maher
-
News & Views |
 Bird flu in mammals
Bird flu in mammals
An engineered influenza virus based on a haemagglutinin protein from H5N1 avian influenza, with just four mutations, can be transmitted between ferrets, emphasizing the potential for a human pandemic to emerge from birds. See Letter p.420
- Hui-Ling Yen
- & Joseph Sriyal Malik Peiris
-
News |
 Mutant-flu paper published
Mutant-flu paper published
Controversial study shows how dangerous forms of avian influenza could evolve in the wild.
- Ed Yong
-
Letter
| Open Access Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets
Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets
Only four mutations in H5N1 HA are required to enable ferret-to-ferret transmission of a reassortant virus containing the H5 HA and the remaining seven gene segments from a human pandemic H1N1 influenza virus.
- Masaki Imai
- , Tokiko Watanabe
- & Yoshihiro Kawaoka
-
News |
 Mutant-flu researcher plans to publish even without permission
Mutant-flu researcher plans to publish even without permission
Virologist plans to defy Dutch government over export permit requirement for avian flu paper.
- Declan Butler
-
News |
 Post-mortem on mutant flu
Post-mortem on mutant flu
Virus papers get green light but controversy highlights lack of global rules on biosafety.
- Declan Butler
-
News |
 Mutations behind flu spread revealed
Mutations behind flu spread revealed
Details of one of two controversial mutant flu papers discussed in London.
- Ed Yong
-
News |
 Moving towards a universal flu vaccine
Moving towards a universal flu vaccine
Israeli company reports good results with supplemental shot in elderly.
- Amber Dance
-
Editorial |
Under surveillance
Global systems for monitoring threats from flu need a radical overhaul.
-
Comment |
 How to track a flu virus
How to track a flu virus
Four experts pinpoint ways to improve monitoring of H5N1 avian influenza in the field.
-
News |
 Flu surveillance lacking
Flu surveillance lacking
Nature analysis highlights need for international strategy to watch for pandemic threats.
- Declan Butler
-
Letter |
 IFITM3 restricts the morbidity and mortality associated with influenza
IFITM3 restricts the morbidity and mortality associated with influenza
Interferon-inducible transmembrane (IFITM) protein 3 is shown to be an innate defence mechanism against viral infection in vivo; furthermore, a subset of the patients hospitalized during the H1N1 2009 pandemic carried a variant form of the IFITM3 gene.
- Aaron R. Everitt
- , Simon Clare
- & Paul Kellam
-
News & Views |
 Influenza's tale of tails
Influenza's tale of tails
Epigenetics is a hot new research field, but it seems that the influenza virus already has it figured out. By mimicking epigenetic regulation in human cells, one flu strain suppresses the expression of antiviral genes. See Article p.428
- Alexei L. Krasnoselsky
- & Michael G. Katze
-
Article |
 Suppression of the antiviral response by an influenza histone mimic
Suppression of the antiviral response by an influenza histone mimic
The H3N2 influenza virus immunomodulatory protein NS1 carries a sequence that mimics the histone H3 tail; this sequence interferes with the host antiviral response via binding to the cellular regulator of RNA elongation, hPAF1C.
- Ivan Marazzi
- , Jessica S. Y. Ho
- & Alexander Tarakhovsky
-
-
Research Highlights |
 Bats can carry flu too
Bats can carry flu too
-
News Explainer |
 The risks and benefits of publishing mutant flu studies
The risks and benefits of publishing mutant flu studies
Research describing two mutant strains of H5N1 avian influenza that spread between mammals is likely to be published in its entirety. Nature examines the controversial decision.
- Ed Yong

 Necroptosis blockade prevents lung injury in severe influenza
Necroptosis blockade prevents lung injury in severe influenza